Preparation of Alcohols from Alkyl Halides
Alcohols can be prepared by substitution reactions using hydroxides and substrates with good leaving groups, such as alkyl halides.
Primary substrates react by the SN2 mechanism, while tertiary substrates undergo SN1 reactions when strong and weak nucleophiles are used, respectively:
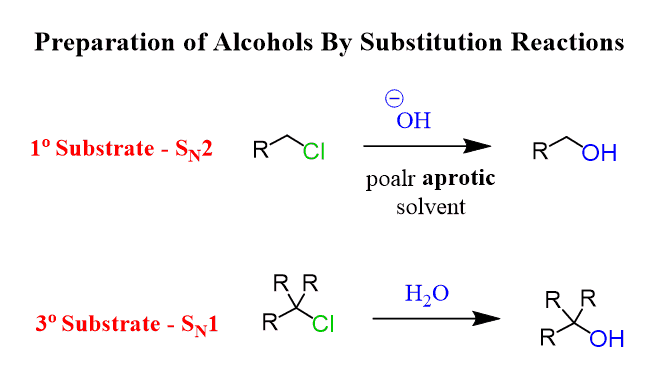
🟢 Check this article on converting Alkyl Halides to Alcohols for more examples, mechanisms, and practice problems.
Preparation of Alcohols from Alkenes
Another way of preparing alcohols is the hydration of alkenes. Remember, alkenes are converted to alcohols by acid-catalyzed hydration, which proceeds with Markovnikov’s rule or by oxymercuration-demercuration, which is a useful method if the substrate is susceptible to carbocation rearrangements.
The hydroboration-oxidation, on the other hand, can be used to prepare alcohols by an anti-Markovnikov addition of water:

🟢 Check the article on converting Alkenes to Alcohols for more examples, mechanisms, and practice problems.
Preparation of Alcohols by the Grignard Reaction
The Grignard reaction can also be used to prepare alcohols from carbonyl compounds such as aldehydes, ketones, esters, epoxides, and acid chlorides:

🟢 Refer to this post for more examples and practice problems on the Grignard reaction, refer to this post.
Check this 66-question, Multiple-Choice Quiz with a 2-hour Video Solution on the naming, preparation, and reactions of Alcohols.
Alcohols Quiz – Naming, Preparation, and Reactions


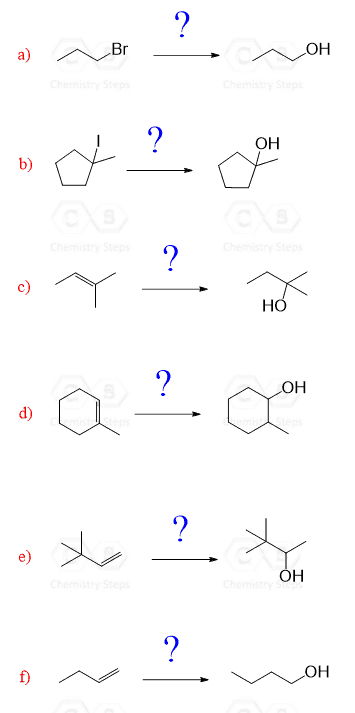
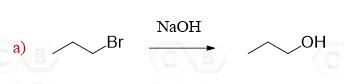
Hi, so I am wondering why you cannot do a Oxymerc. reaction in d and/or f?
Hello,
The oxymercuration reaction is used for a Markovnikov addition primarily to avoid possible rearrangements that could occur in acid-catalyzed hydration. The drawback is the toxicity of mercury which can be ignored on paper.