The main difference of the Grignard reaction compared to everything else you learn in the reactions of alcohols is that it is another way of making a new C-C bond and extending the carbon chain of the molecule.
The most common Grignard reaction is carried out with aldehydes and ketones forming secondary and tertiary alcohols and with esters forming tertiary alcohols:

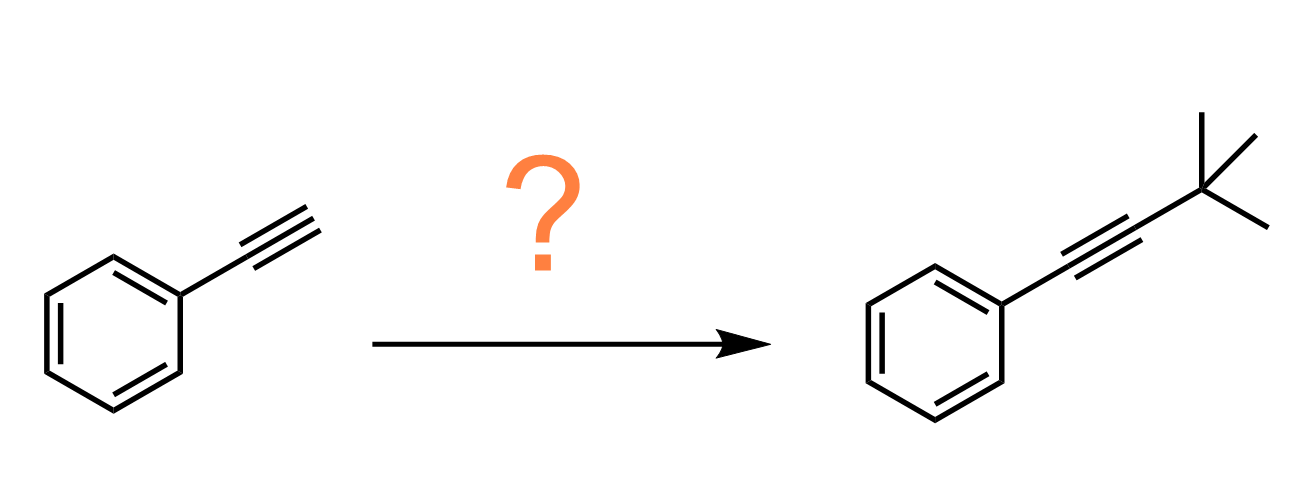
In a standard undergraduate curriculum this is the second reaction for making carbon-carbon bonds after the alkylation of terminal alkynes:
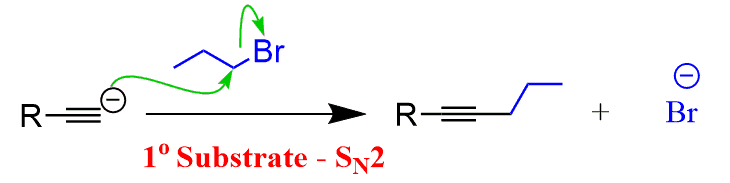
These two approaches of extending the carbon chain can be used interchangeably for preparing carbonyl compounds. While the Grignard reaction requires oxidation of the secondary alcohol into the corresponding ketone, the triple bond can directly be converted into a ketone or an aldehyde using the hydroboration-oxidation of alkynes:

The advantage of the Grignard reaction is the regioselectivity of preparing ketones. Remember, the acid-catalyzed hydration or hydroboration-oxidation of internal alkynes results in a mixture of two ketones:
Example: Propose an efficient synthesis for the following ketone:
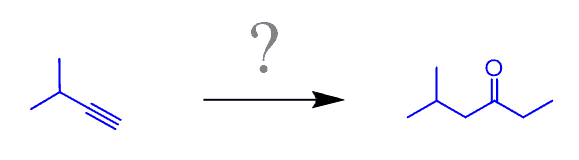
First, number the carbon atoms. This will always help you tremendously! If you see an increase in the number of carbon atoms, think of the alkylation of terminal alkynes and the Grignard reaction.
There are two more carbons in the product than in the starting alkene. Therefore, we can deprotonate the acidic proton of the alkyne and react it with ethyl iodide to perform an SN2 reaction and extend the carbon chain:

The resulting alkyne can be converted into the desired ketone by either acid-catalyzed hydration or hydroboration-oxidation. The problem, however, is the formation of two ketones as there is no regioselectivity of hydration of internal alkynes.
So, let’s try to see if the Grignard reaction will be a more efficient way of preparing the target ketone.
The alkyne is functionalized towards the less-substituted carbon on the right. That is why you need to use the hydroboration-oxidation to convert it into the corresponding aldehyde.
The Grignard reaction with ethyl magnesium bromide followed by the oxidation of the secondary alcohol will produce the target ketone in good regioselectivity:
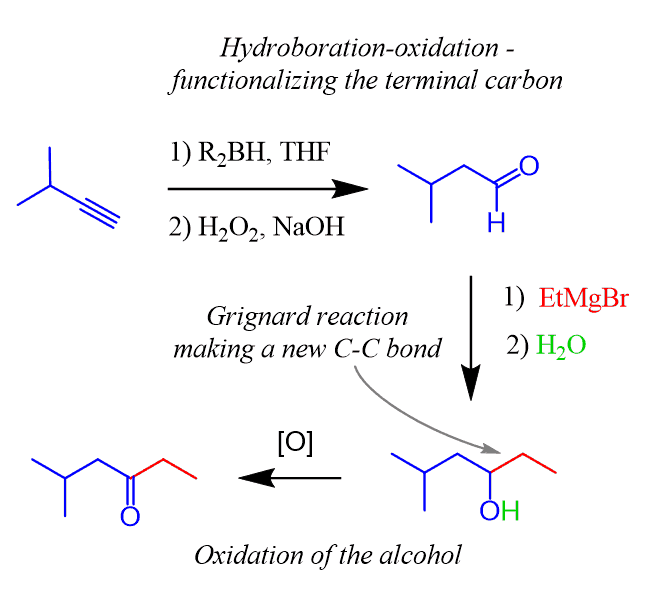
Notice that the secondary alcohol can be oxidized by any mild or strong oxidizing agent since there is no concern about over-oxidizing it to a carboxylic acid.
Therefore, in the following practice problems of Grignard reaction, anytime you see the [O] notation, interpret it as “any oxidizing agent” (strong or mild) such as Na2Cr2O7/H2SO4, KMnO4, PPC, DMP, Swern etc.
For a selective oxidation of a primary alcohol to the corresponding aldehyde, we will use PPC.
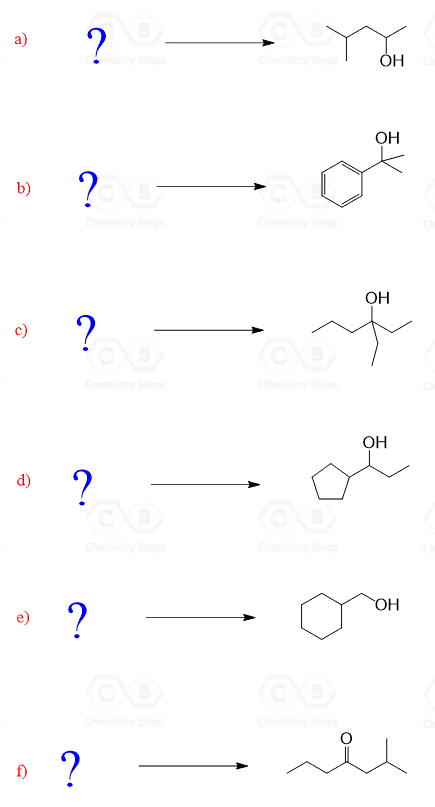
You can first practice identifying the reactants in Grignard reactions. Each synthesis has more than one way of combining the corresponding carbonyl or another electrophile with a Grignard reagent.

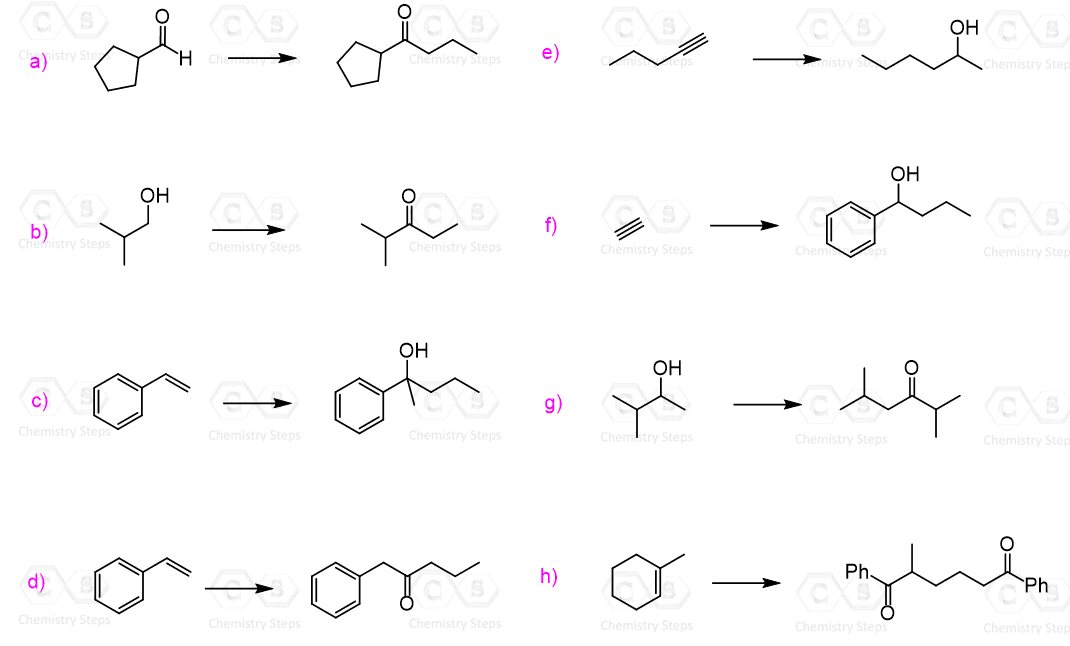
 The product has three more carbons than the starting aldehyde. Therefore, you need to think of making new C-C bonds to extend the carbon chain. So far, you have probably covered only two reactions that allow making new carbon-carbon bonds: the
The product has three more carbons than the starting aldehyde. Therefore, you need to think of making new C-C bonds to extend the carbon chain. So far, you have probably covered only two reactions that allow making new carbon-carbon bonds: the
For C, wouldn’t the first reaction, with H3O+ rearrange to form a more stable carbocation? Would treating the alkene with oxymercuration/demercuration work better?
That’s a good habit to keep rearrangements in mind! However, in this case, it won’t happen since there is no hydrogen on the carbon connected to the ethyl group. And even if there was one, or we could get a tertiary carbocation in the ring, it still wouldn’t shift since the resulting tertiary carbocation would come at the price of breaking the aromaticity. Aromatic compounds have additional stability and breaking the double bonds in the ring is energetically not favorable.
Oh! I see that now. Thanks for the clarification.