1.
Predict the kinetic or thermodynamic product of electrophilic addition to each conjugated diene:
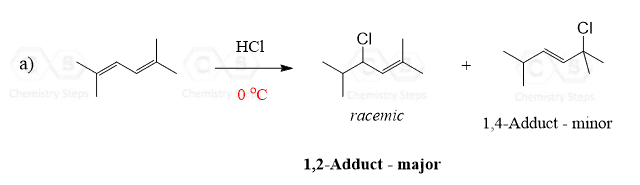
Answer
At low temperatures, the kinetic product formed via 1,2-addition leads to the major product:
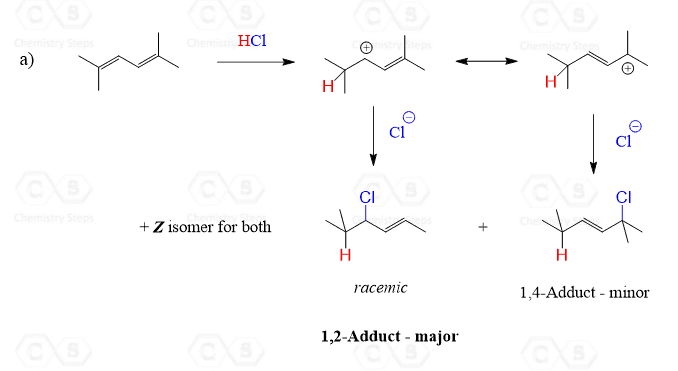
The resonance structures leading to each product are shown in the solutions.
Solution
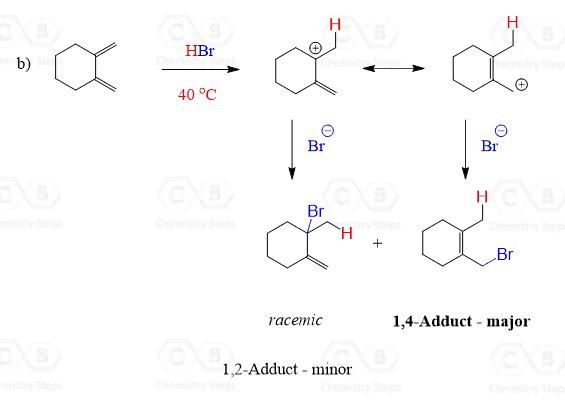
The diene in this reaction is symmetrical and therefore protonation of only one of the two double bonds is considered for predicting the structure of organic products.
2.

Answer
At elevated temperatures, the thermodynamic product formed via 1,4-addition leads to the more substituted alkene as the major product: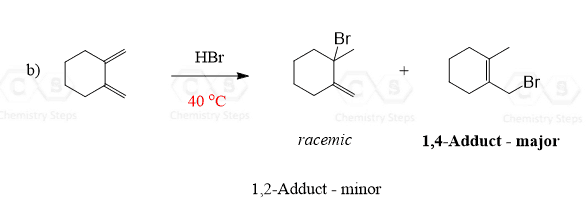
Solution
The diene in this reaction is symmetrical and therefore protonation of only one of the two double bonds is considered for predicting the structure of organic products.