We talked about aromatic and antiaromatic compounds which are recognized based on the Hückel’s rule.
In short, the only way aromatic and antiaromatic compounds differ is the number of electrons they have in the conjugated system. All the other criteria – being cyclic, planar, and fully conjugated are a must for both categories:

Nonaromatic Compounds
There is also the third class of compounds we need to discuss: These are the nonaromatic or not aromatic compounds. As the name suggests, nonaromatic compounds have really nothing to do with the aromaticity (well, almost).
For example, is this compound aromatic?
![]()
You’d be like, how is that supposed to be aromatic?
And that is the idea, nonaromatic compounds are the ones not related to the aromatic and antiaromatic compounds:

In fact, if any of these factors; cyclic, planar, fully conjugated does not match – the compound is said to be nonaromatic.
Sometimes though, it may not be so obvious because some molecules look like they are aromatic or antiaromatic based on the factors mentioned above.
A good example is cyclooctatetraene which we have talked about earlier:

It looks like a perfect candidate to be named antiaromatic – cyclic, planar, fully conjugated, and 8 electrons (4n formula for antiaromatic compounds).
Remember, however, what we said about antiaromatic compounds – if the molecule has a way of avoiding being antiaromatic, it will do it.
So, how does cyclooctatetraene avoid being antiaromatic?
The answer is it adopts a geometry that is not planar (flat):

The double bonds are not in resonance as the p orbitals of adjacent double bonds cannot overlap. They are like separate alkenes and that is why cyclooctatetraene undergoes regular electrophilic addition reactions that alkenes do at even lower temperatures since getting rid of a double bond releases some of the strain associated with this geometry:

Another example is [10]-annulene which has 10 π electrons thus satisfying the Huckel’s 4n+2 rule.

However, it is nonaromatic because it cannot adopt a planar geometry. The problem is the lack of space for the inner hydrogens:

If the carbons were connected thus removing these hydrogens, we’d have naphthalene which is now planar, and it is aromatic:

Notice that a lot of compounds can be nonaromatic because they fall out of the aromatic-antiaromatic business as soon as only one of the criteria -cyclic, planar, fully conjugated is not met.
The antiaromatic, on the other hand, is very specific (and close to the aromatic) – it must meet all these criteria but instead of having 4n+2 electrons, it has 4n. And this makes antiaromatic compounds very rare because that specific combination is energetically unfavorable.
So, let’s put a little summary flowchart for identifying aromatic, antiaromatic, and nonaromatic compounds:

A great tool for distinguishing aromatic, antiaromatic, and nonaromatic compounds is the Frost circle which relies on having unpaired electrons in nonbonding orbitals for predicting antiaromatic nature. results in nonaromatic compounds.
For example, azepine is oxepin are predicted to be antiaromatic by Huckel’s rule and unpaired electrons in the nonbonding orbitals:
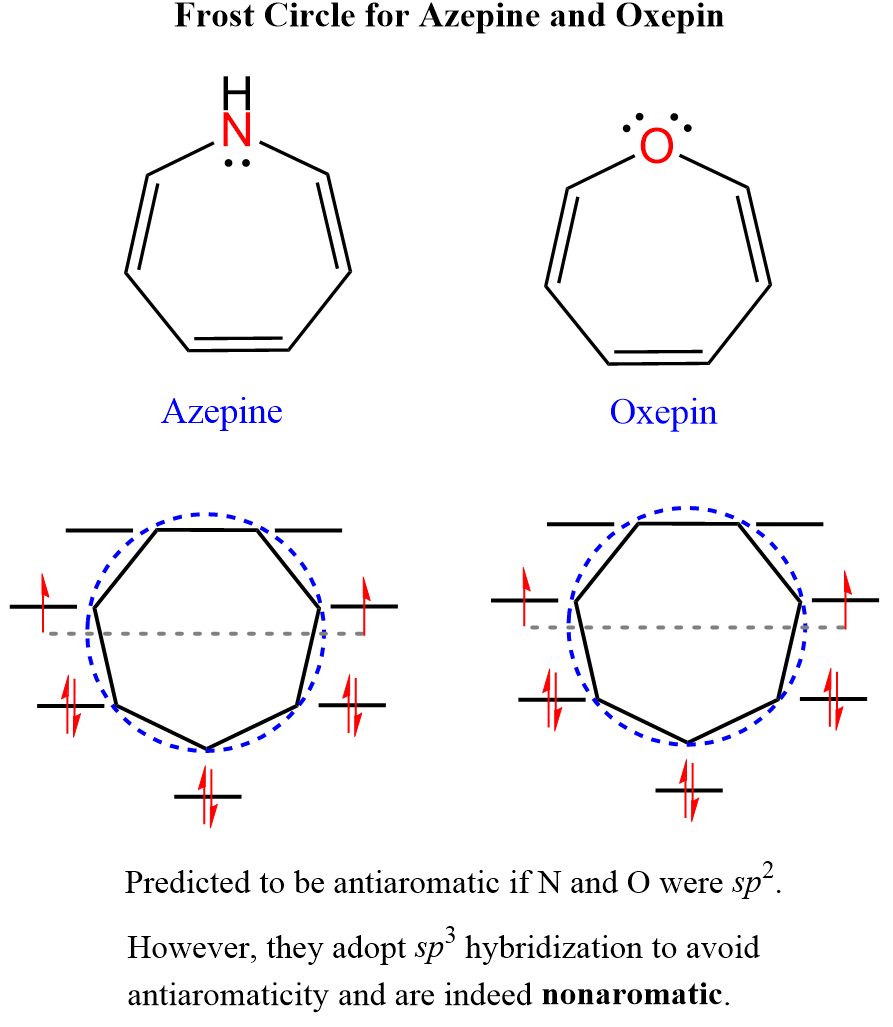
However, the oxygen and nitrogen adopt an sp3 hybridization making them nonaromatic as otherwise, being sp2 would make one of its lone pairs part of a fully conjugated cyclic system of 8 electrons making the molecule antiaromatic.
For larger cycles, commonly referred to as annulenes, there is also a post you can read:
And lastly, keep in mind that being aromatic is not a requirement for stability. Not being aromatic does not necessarily make the molecule unstable. And even though there might be nonaromatic compounds that are particularly stable, as a general statement it would only relate to antiaromatic compounds.
Below are some examples to practice determining compounds as aromatic, nonaromatic, or antiaromatic.



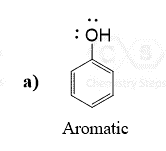
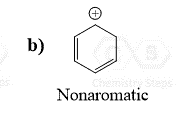

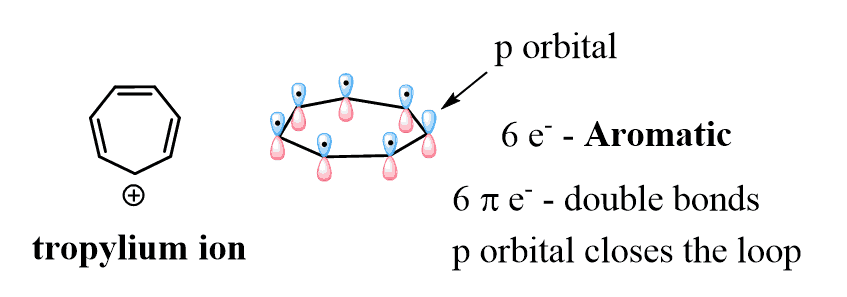
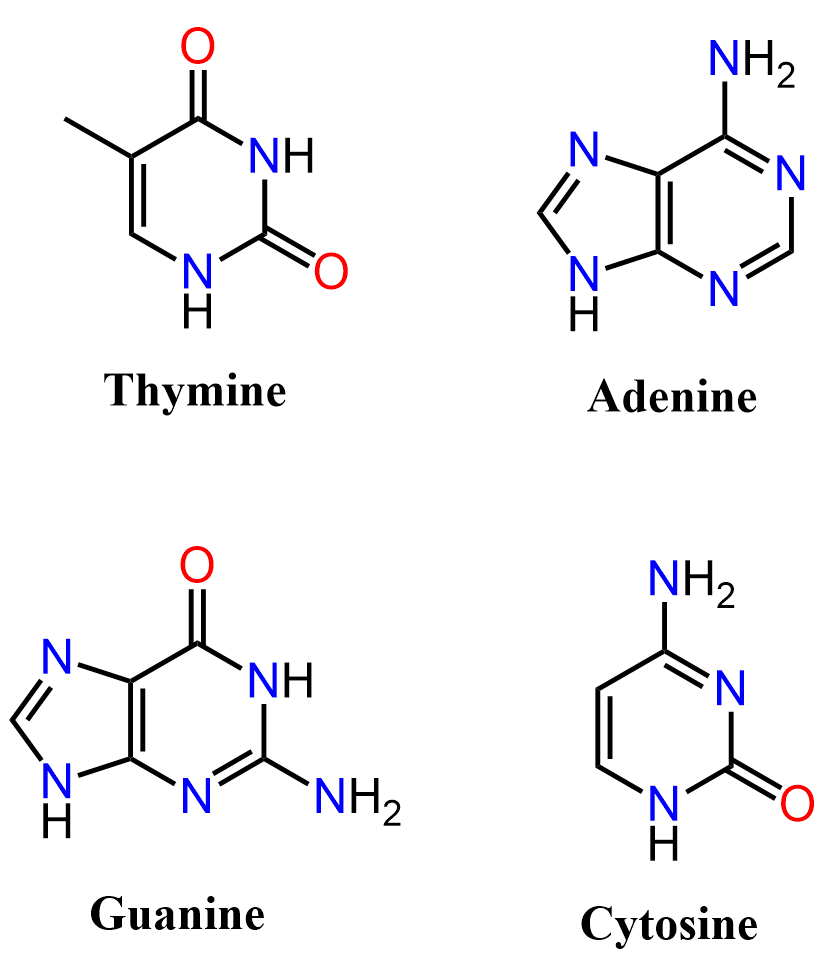
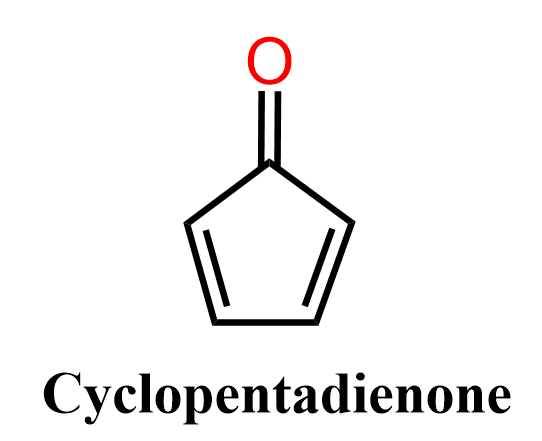
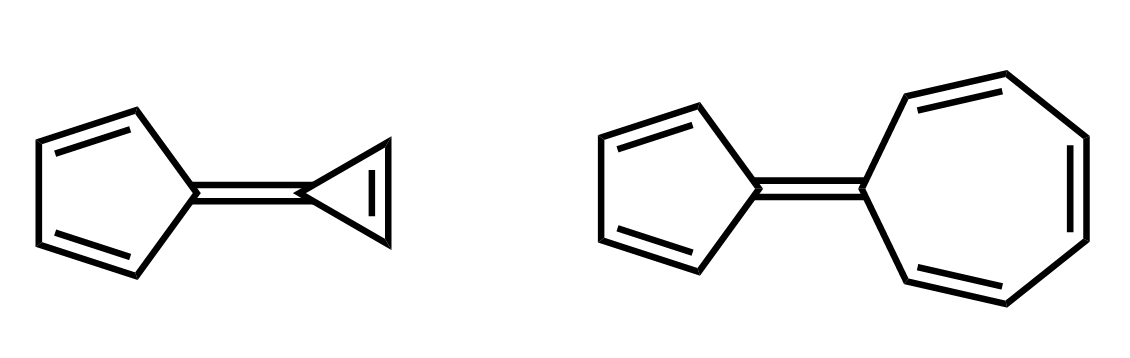
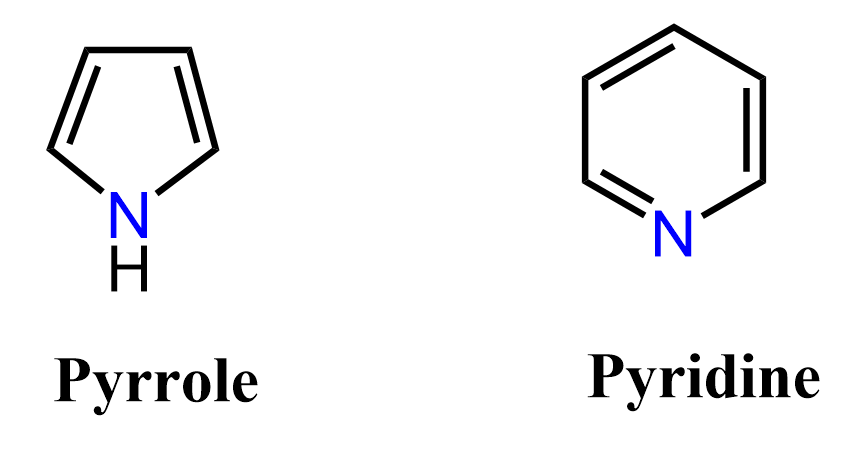
It’s really interesting
helpful, thank you
thank you so much!!
This is the best explanation of aromatic antiaromatic principle, and the practice problems are immensely helpful. I am amazed how you make it so detailed but succinct at the same time. Thank you so much.