Benzene has 3 π bonds and, as expected, shows some similarities to alkenes in being reactive towards electrophilic species. However, there are two key differences between their reactions with electrophiles.
First, benzene is very stable and thus less reactive. Second, unlike the alkenes, it undergoes an electrophilic substitution and not an electrophilic addition reaction:

The first difference of benzene being less reactive brings the need for using a Lewis acid, such as FeBr3, which turns the Br2 into a stronger electrophile and makes the reaction possible. We will see how that works next.
The second difference is that the Br in the electrophilic aromatic substitution reaction replaces the hydrogen, while both hydrogens are still there when they are on the alkene. And in fact, this is still related to the stability of the aromatic ring. Even though the reaction goes through an intermediate where the aromaticity is broken, it still ends up restored because that brings a lot of stability and energetically is very favorable.
The Mechanism of Electrophilic Aromatic Substitution
Regardless of what electrophile is used, the electrophilic aromatic substitution mechanism can be divided into two main steps.
In step 1, the π electrons of benzene attack the electrophile, which takes two electrons of the six-electron aromatic system. This forms a σ bond between one carbon atom of the benzene ring and the electrophile. Because of the new sigma bond formed, this intermediate is called a sigma complex.

Notice that this breaks the highly stable aromatic system since in the intermediate arenium ion, the carbon connected to the electrophile becomes sp3 hybridized and, therefore, cannot be part of the conjugated π bond system. This energetically unfavorable process of interrupting aromaticity is the slow, rate-determining step of the reaction.
On the other hand, the arenium ion is not the worst carbocation you will ever see. It is secondary; there are two conjugated double bonds, which in turn are conjugated with the empty p orbital of the positively charged carbon. It has three resonance forms, where the positive charge appears on three carbons, and the resonance hybrid can be shown with these carbons having a partial positive charge:

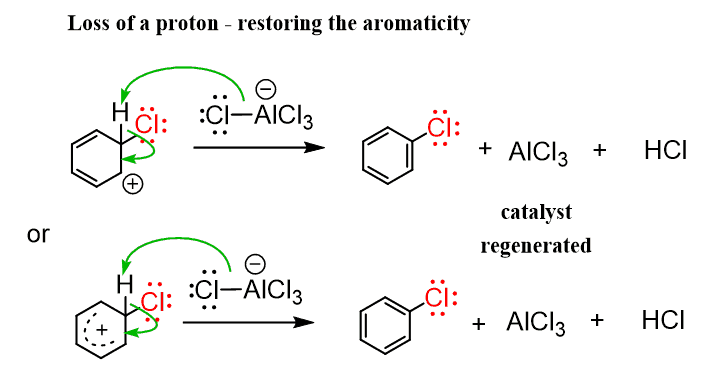
In the second step, the hydrogen on the sp3-hybridized carbon is removed by a counterion/conjugate base, restoring the aromaticity to the ring:
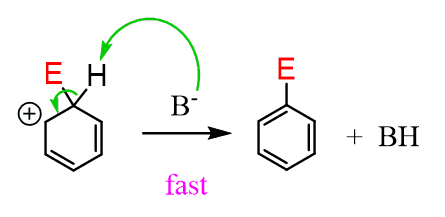
The deprotonation is the driving force of the reaction, making it energetically possible to proceed. The activation energy of this step is a lot smaller, and the reaction occurs very fast:

Now, let’s look at specific examples.
Halogenation of Benzene
Benzene only reacts with bromine and chlorine in the presence of Lewis acids as they coordinate to the halogens and generate strong electrophilic species. The Lewis acids are usually aluminum chloride (AlCl3) or iron chloride (FeCl3) used for the chlorination, and iron bromide (FeBr3) for the bromination of the aromatic ring:
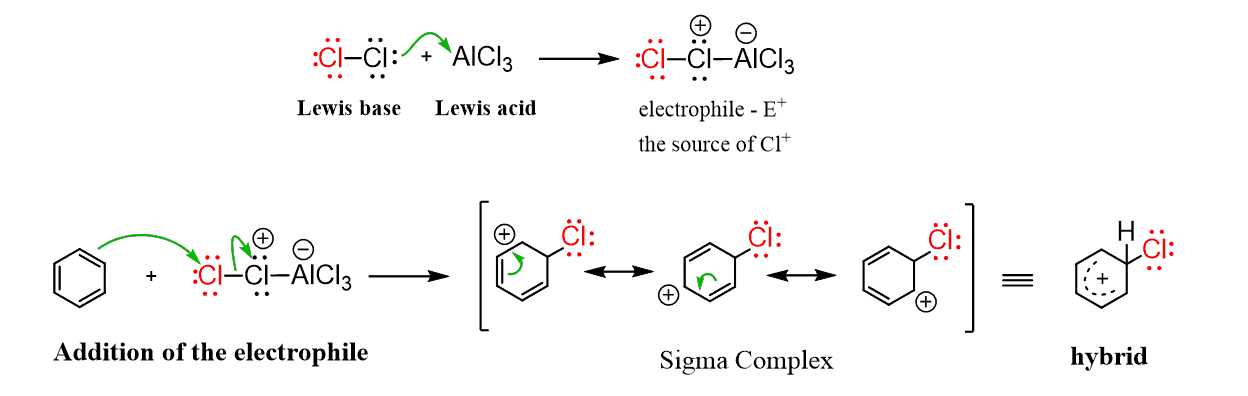
Once the electrophile is formed, it follows the same general mechanism as we have discussed earlier. First, the addition of the electrophile, forming the sigma complex which is then deprotonated by –AlCl4.
In the same way, FeBr3 is used as the Lewis acid activator for generating the source of Br+. The rest of the mechanism is identical to what we saw for the chlorination of benzene.
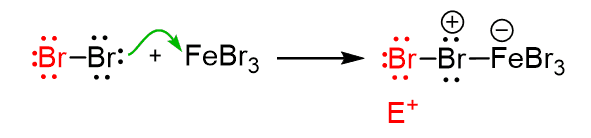
Sometimes, Fe may be shown instead of FeBr3, but don’t worry, it is the same thing as Fe as it reacts with Br2 to form the catalyst FeBr3 in situ (in the reaction mixture).
Iodination of Benzene
Iodine is unreactive under identical conditions, and the iodination of benzene is achieved in the presence of an oxidizing agent such as nitric acid or a mixture of hydrogen peroxide and sulfuric acid. They oxidize the I2 to I+ and after this, it follows the standard mechanism of the electrophilic aromatic substitution.
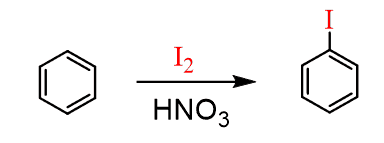
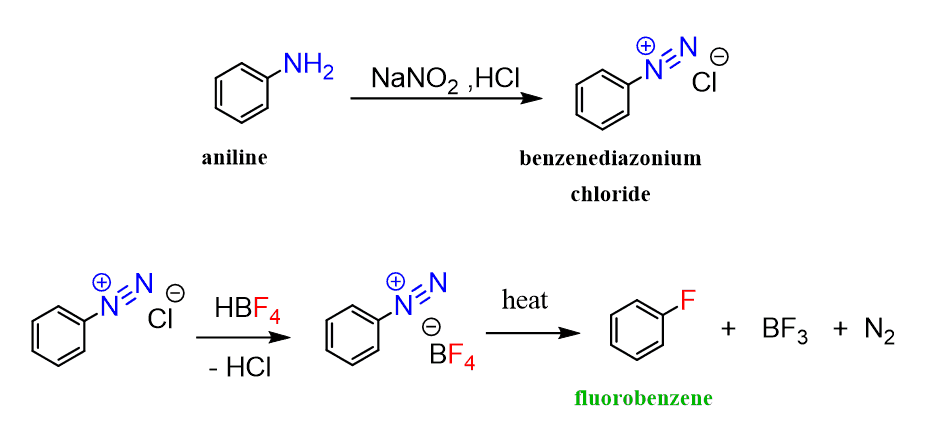
Fluorination of Benzene
Fluorination of benzene, on the other hand, is a violent reaction and cannot be achieved directly. Plus, handling F2 is not really what you want to do unless you absolutely have to, and are trained to do so.
Instead, it is done by converting benzene into an arenediazonium salt which is then replaced by fluorine by reacting it with fluoroboric acid (HBF4). This is the Schiemann reaction.
Nitration of Benzene
The electrophile in the nitration of benzene is the +NO2 (the nitronium ion), which is formed by protonation of HNO3 by H2SO4 (yep, sulfuric acid is powerful). The rest is according to the general mechanism of electrophilic aromatic substitution:
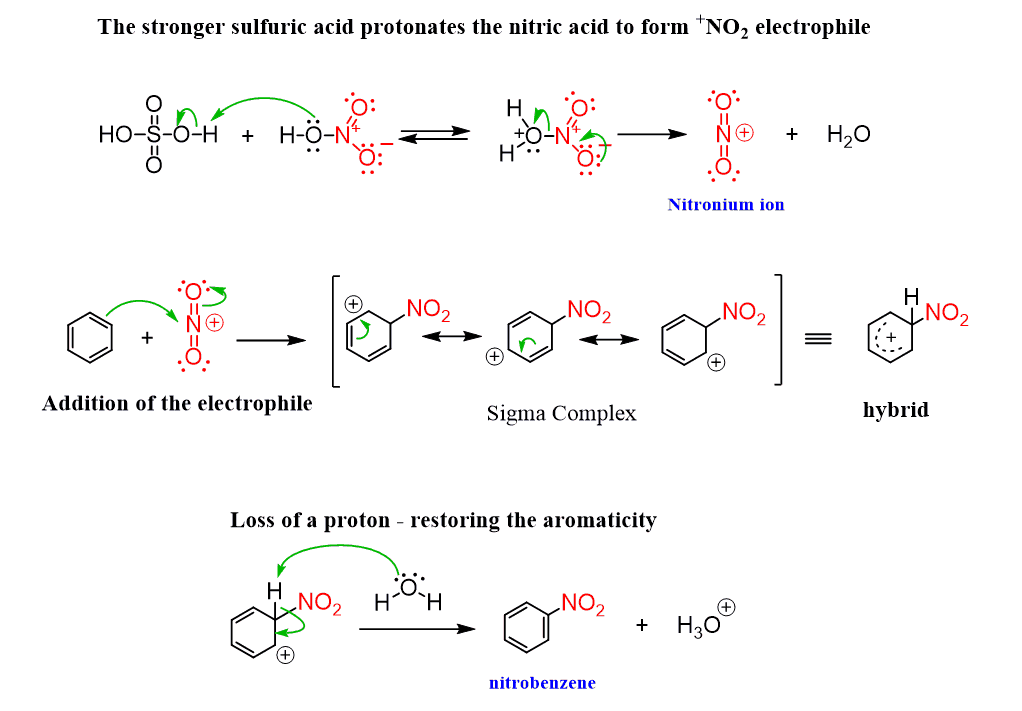
The nitration of benzene is an important reaction since nitrobenzene is an essential precursor for the synthesis of aniline, which is used in many other reactions, including the one we have just seen for the synthesis of fluorobenzene.
Sulfonation of Benzene
Benzene can be converted into benzenesulfonic acid by reacting it with fuming sulfuric acid, which is prepared by adding sulfur trioxide (SO3). The electrophile in this reaction is the sulfonium ion (+SO3H) that forms when concentrated sulfuric acid reacts with SO3.
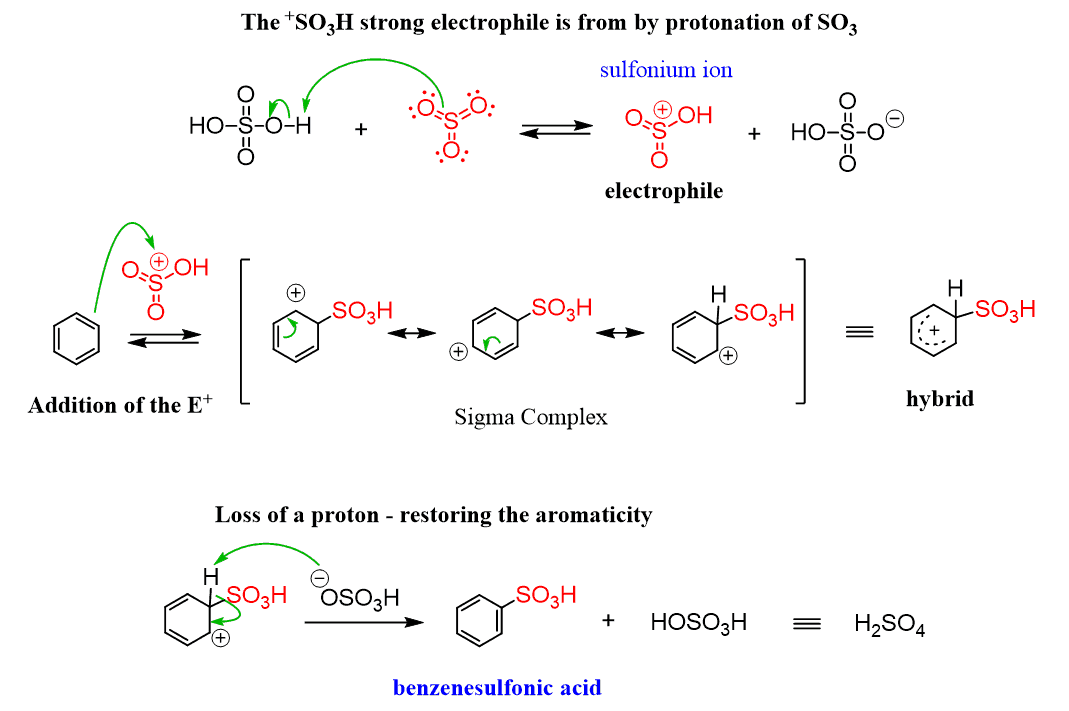
One interesting feature and advantage of the sulfonation is that it is a reversible reaction:
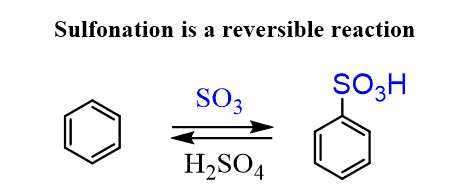
Depending on your needs, you may shift the equilibrium to either side. If you need a sulfonation of the aromatic ring, then use a concentrated solution of H2SO4.
If you need to remove the sulfonate group (and you may wonder, why you’d do that), then a dilute solution of H2SO4 should be used. This selective placing of the SO3 group on the aromatic ring is used as a protecting group, to temporarily block its position from other electrophiles, or as a directing group, which we will discuss in the following posts.
Check this 60-question, Multiple-Choice Quiz with a 1.5-hour Video Solution covering the naming and electrophilic aromatic substitution reactions.
Aromatic Compounds


Very good compiling for God’s sake
Good content