Aryl diazonium salts can be used as an alternative to the standard electrophilic aromatic substitution reactions for preparing aromatic compounds as well as for synthesizing compounds that cannot be prepared directly from benzene.
Before going into the details, here is the summary of the aryl diazonium salts reactions we will talk about today:

Although Sandmeyer reactions (discovered in 1884 by Swiss chemist Traugott Sandmeyer) originally referred only to the conversion of arene diazonium salts to aryl halides using copper(I) salts, it is now often used more broadly to describe a variety of transformations involving arene diazonium salts.
And now, let’s discuss these reactions. First, what are aryl diazonium salts?
Arenediazonium salts are very important and useful intermediates in the synthesis of aromatic compounds since they contain one of the best leaving groups, N2, which can be replaced by a variety of other atoms or functional groups:
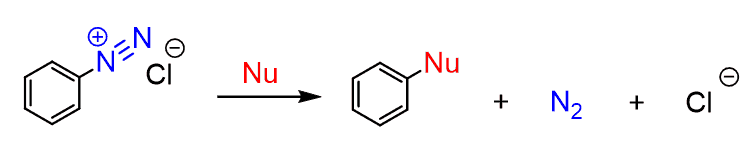
They can be synthesized from benzene according to the following scheme:
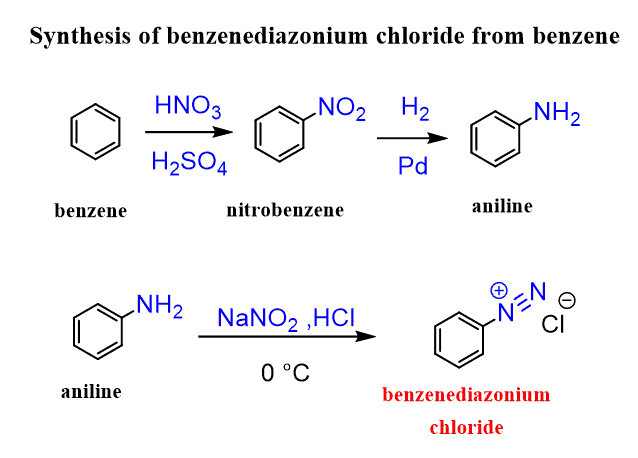
Aniline is reacted with nitrous acid (HNO2) at low temperature since diazonium salts are unstable and are used right after they are prepared. More details about the synthesis of diazonium salts are covered in the amines chapter.
Reactions of Arene Diazonium Salts
Now, remember, low stability also means high reactivity, and that is what makes them useful in the synthesis of many compounds that are otherwise difficult to achieve.
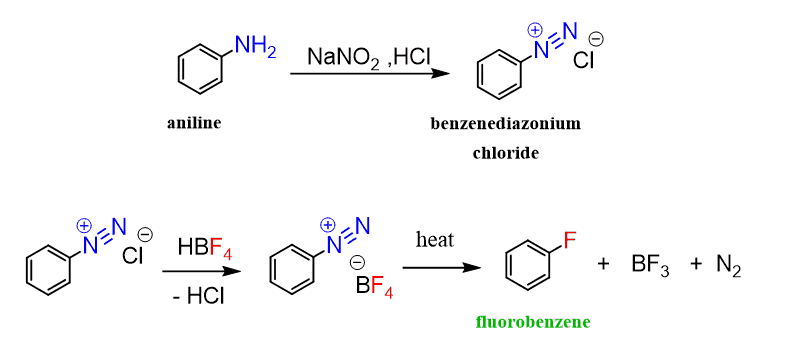
For example, we know that chloro- and bromobenzene can be prepared by halogenation using a Lewis acid catalyst. However, fluorination of benzene is a violent reaction, and fluorobenzene is prepared by first converting the benzene into benzene diazonium chloride, which is then heated with fluoroboric acid (HBF4):
In a similar way, other halogenated rings can also be prepared by the Sandmeyer reaction of an arenediazonium salt with a copper(I) salt:
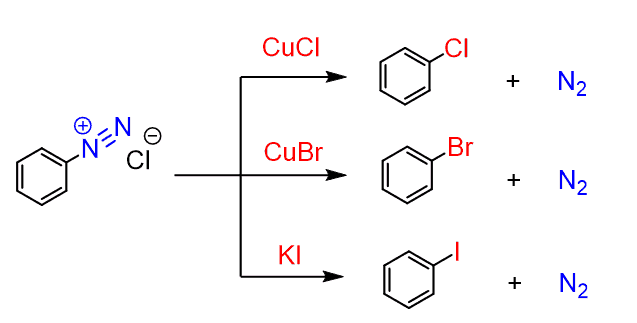
This is an alternative approach for the halogenation with the advantage that it can be used to prepare meta-substituted trihalogenated rings, which is impossible by the standard halogenation.
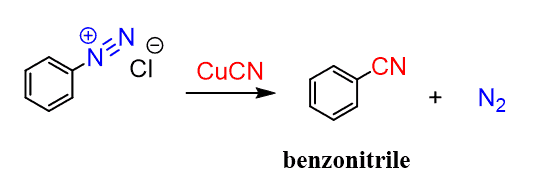
The cyano group can also be incorporated by the Sandmeyer reaction:
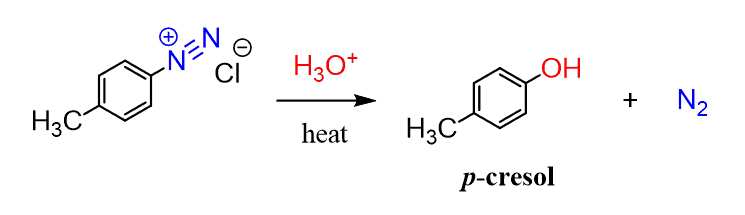
All these reactions can also be done on substituted benzene rings. For example, the hydrolysis of the diazonium salt forms the corresponding phenol. The addition of cuprous oxide can increase the yield of this reaction:
The diazonium group can also be replaced by a hydrogen when reacted with hypophosphorous acid (H3PO2) following a free-radical mechanism:

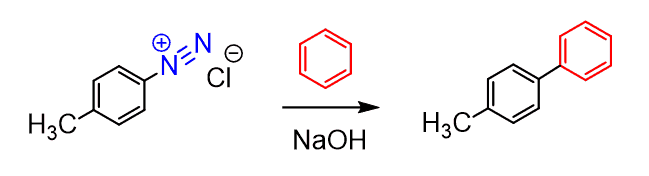
Two aromatic rings can be coupled (connected) by the Gomberg reaction:
Now, going back to the halogenation, why would you say it is impossible to prepare the 1,3,5-tribromobenzene by bromination of the benzene?
How would you prepare it by using a diazonium salt?
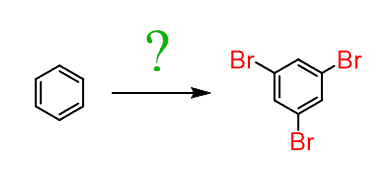
First, let’s mention that 1,3,5-tribromobenzene cannot be prepared by the direct EAS halogenation since halogens are ortho, para-directors and once the first bromine is installed on the ring, it will direct the others to ortho and para positions:

This problem can be overcome by the useful feature of the diazonium salt, which is that it can be removed from the ring once the desired transformation is achieved. And this is the reaction with hypophosphorous acid we discussed above.
So, the strategy is to prepare aniline, brominate it at the ortho and para positions, and finally remove the NH2 group by converting it into a diazonium salt:
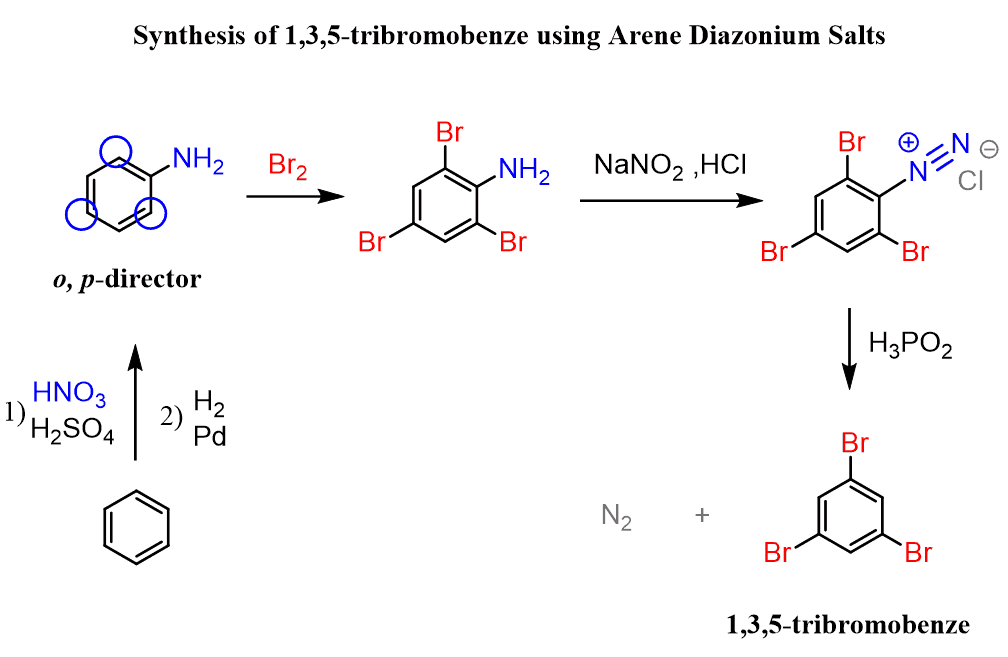
Notice that the bromination of aniline did not require a catalyst because it is a very strong activator, and the reaction is impossible to stop at monobromination even in these conditions.
Arenediazonium Ions as Electrophiles – Azo Coupling
Another interesting feature of arenediazonium ions is their electrophilicity. The positively charged nitrogen makes them susceptible to a nucleophilic attack of activated aromatic rings. The reaction works best with highly activated benzene rings such as phenols, alkoxy benzenes, anilines, and N-alkylanilines:
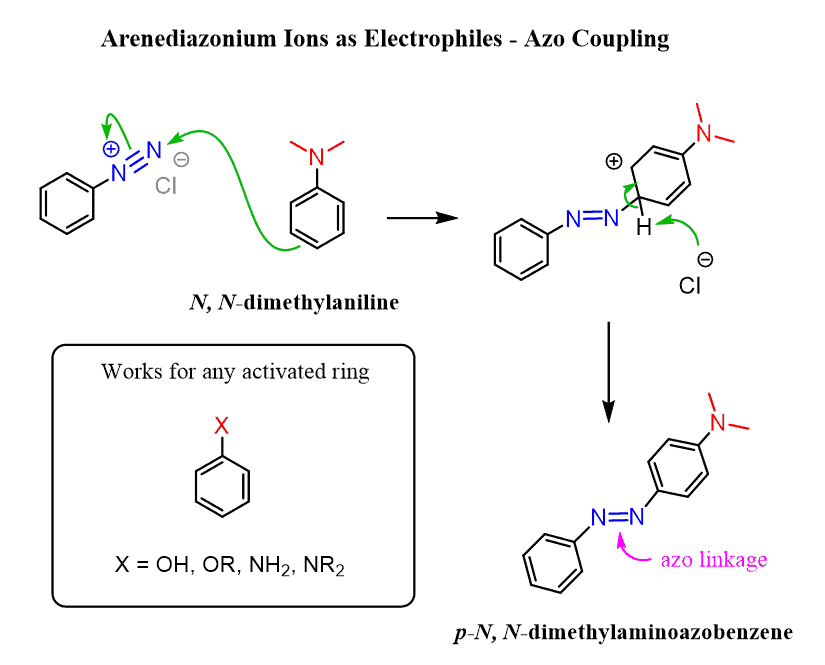
The product of the reaction is called an azo compound, which is colorful due to the extended conjugation. These chromophores are also known as azo dyes and are used to treat clothing, leather, and some foods.
Cis and Trans Isomerism of Azo Compounds
Azo compounds exhibit cis and trans isomerism, but what’s more interesting is that these isomers can be interconverted by UV light – photoisomerization:
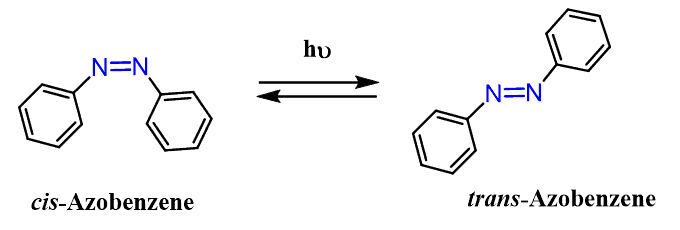
This fascinating feature is at the core of many research groups focused on the development of sensors and nanomachines.

