So far, we’ve seen a variety of nucleophiles in organic chemistry – halides, hydroxides, alkoxides, thiols, etc. Most of these nucleophiles contain heteroatoms like oxygen, nitrogen, or sulfur, but nucleophiles can also be carbon-based, and these are among the most powerful and useful ones in organic synthesis.
In Organic Chemistry 1, the most common carbon nucleophiles you’ll encounter are:
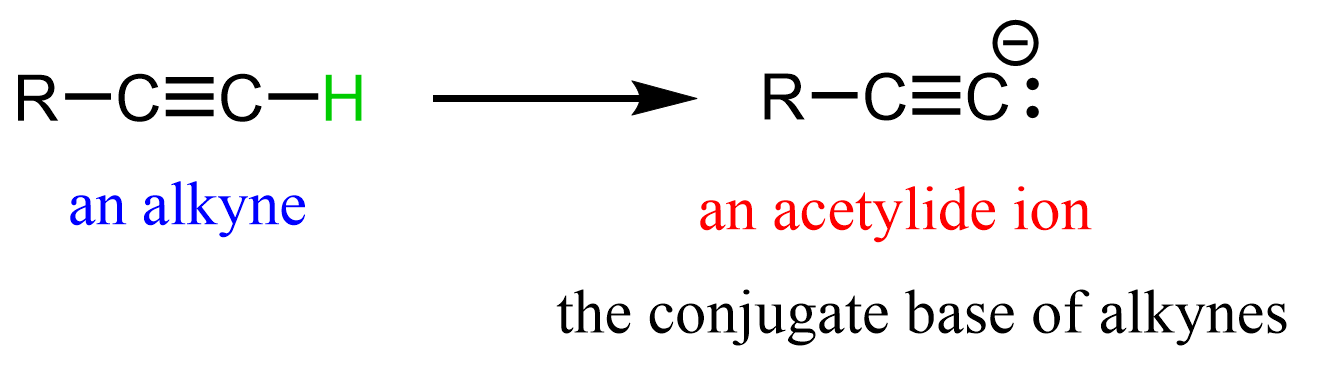
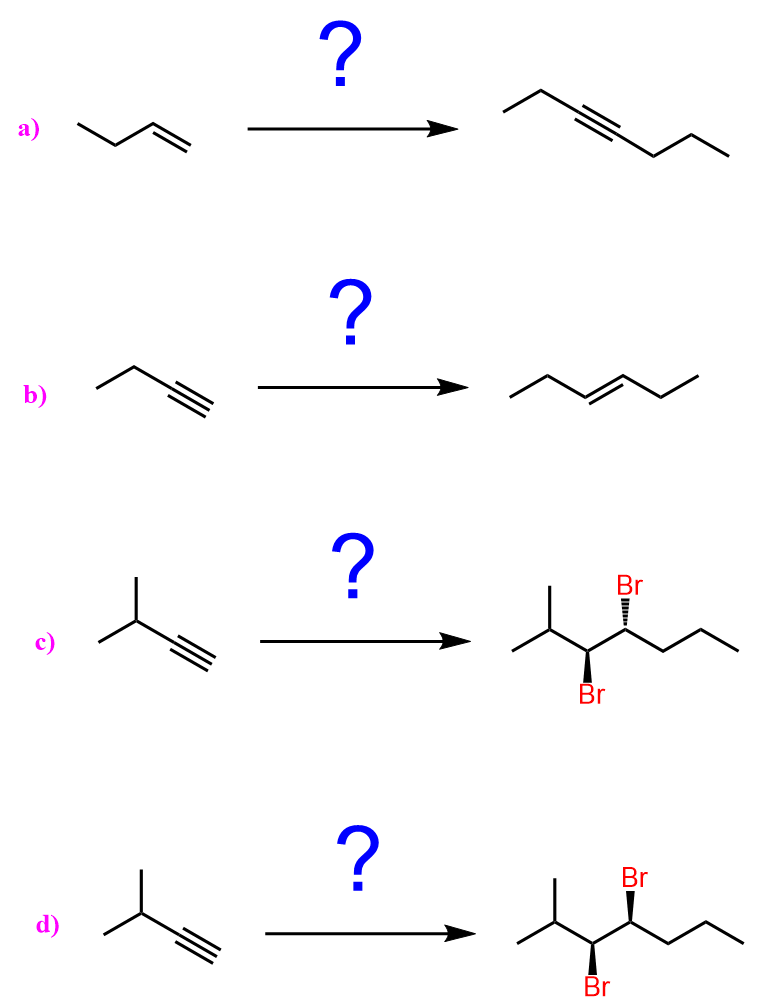
- Acetylide ions (RC≡C⁻) – formed by deprotonating a terminal alkyne with a strong base such as NaNH₂.
- Grignard reagents (RMgX) – typically covered later in the course, often in the chapter on alcohols.

Both of these have a negatively charged carbon that acts as a strong nucleophile, attacking electrophilic carbons like those in alkyl halides or carbonyl compounds.
The Acetylide Ion as a Nucleophile
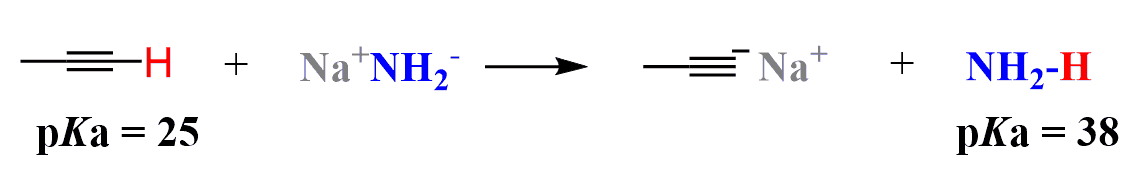
The acetylide ion is formed by removing the acidic proton from a terminal alkyne (RC≡CH) using a strong base such as sodium amide (NaNH₂) or sometimes sodium hydride (NaH).
Of course, this strategy only works with terminal alkynes and not with other hydrocarbons such as alkanes or alkenes because those C–H bonds are much less acidic and very difficult to deprotonate even by very strong bases.
The deprotonation of terminal alkynes works because the hydrogen attached to the sp-hybridized carbon in a terminal alkyne is significantly more acidic (pKa ≈ 25) than the hydrogens on sp² or sp³ carbons.

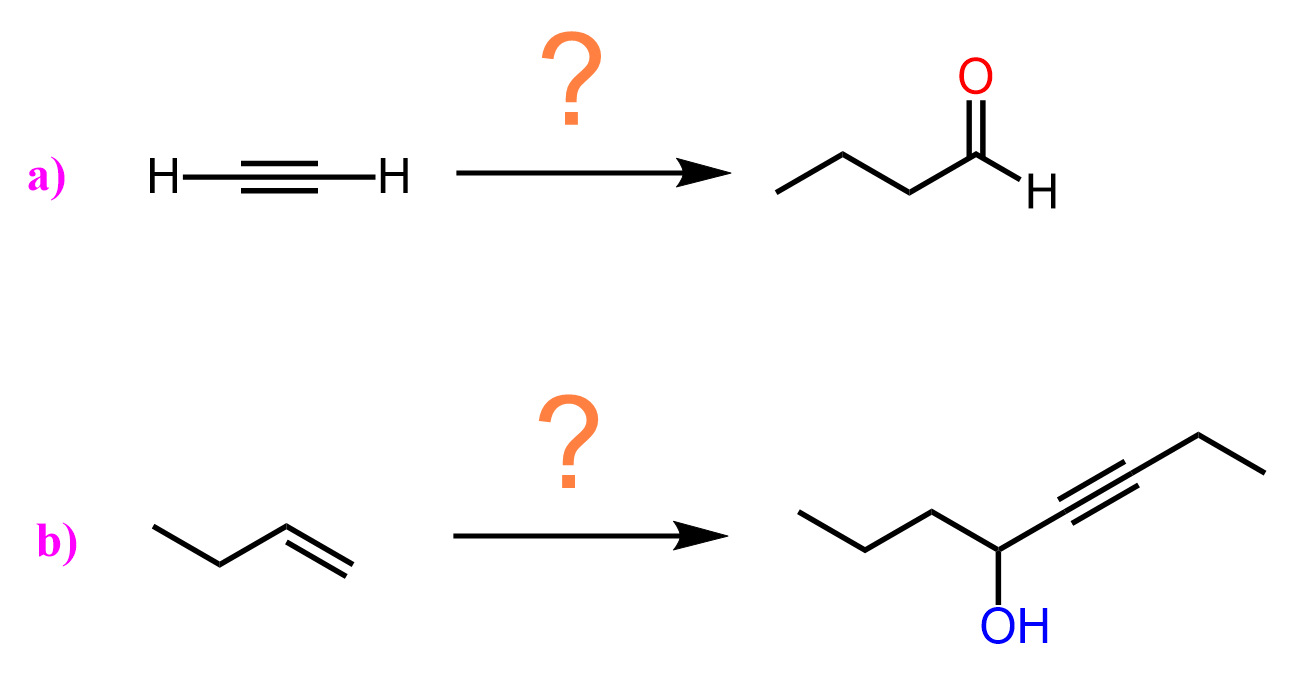
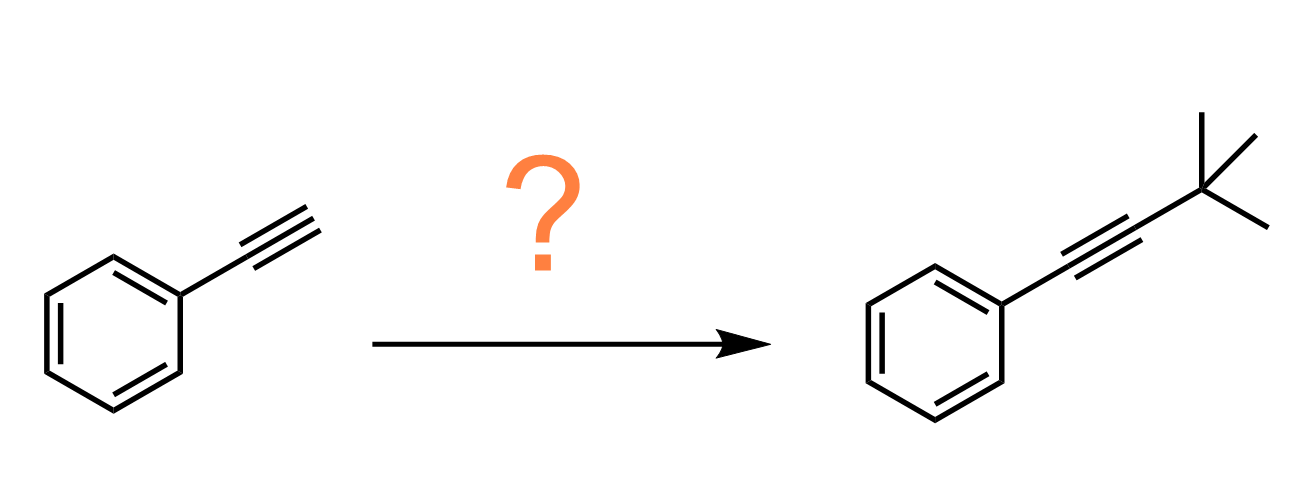
The resulting acetylide ion (RC≡C⁻) is both a strong base and a powerful nucleophile. It can attack electrophilic carbons, especially in primary alkyl halides, to form new carbon-carbon bonds through an SN2 reaction.
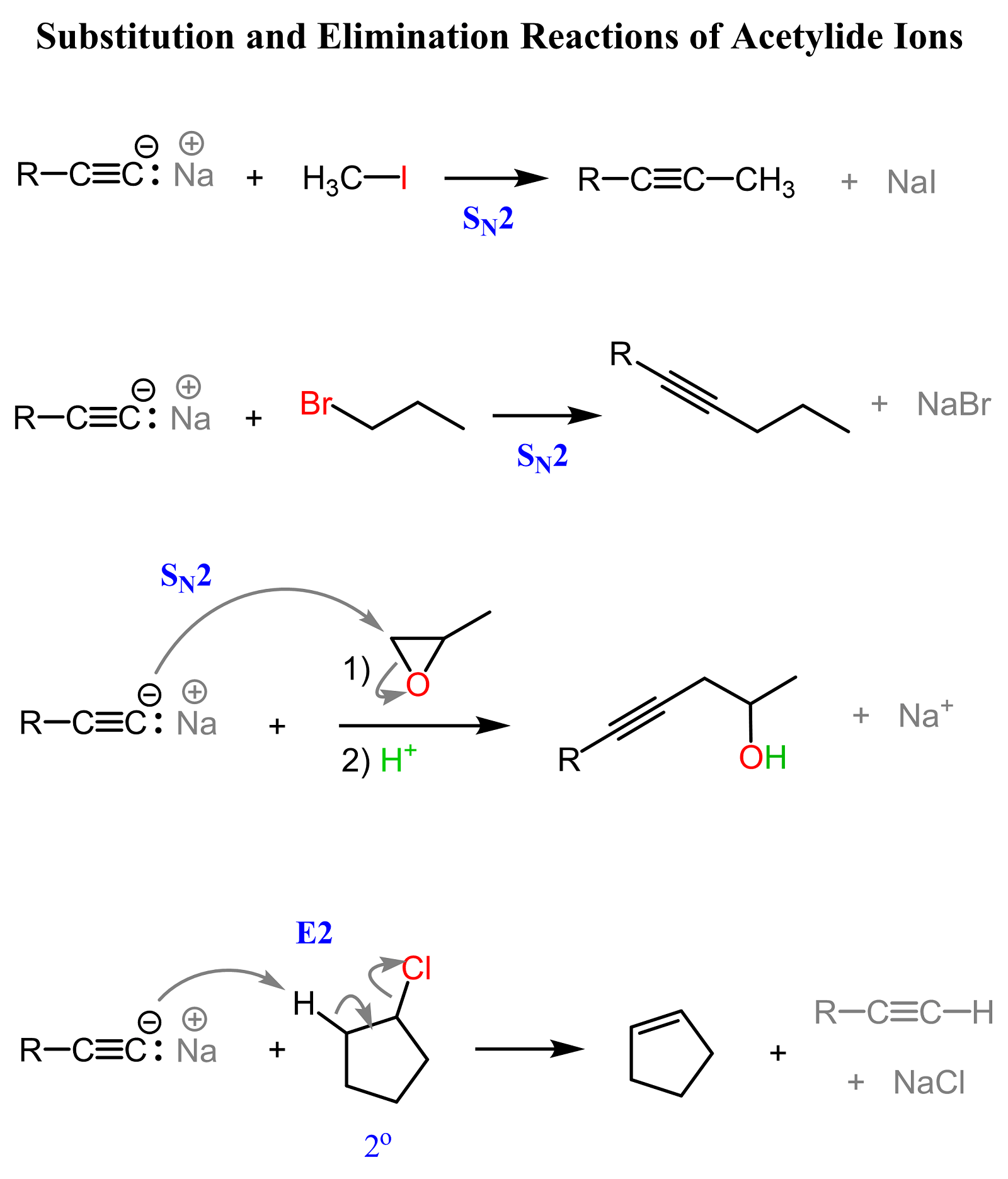
Notice that the SN2 reaction works only with primary alkyl halides because secondary and especially tertiary halides will favor elimination (E2) instead of substitution.
So, always pay attention to the structure of the alkyl halide when designing a synthesis involving SN2 reactions.
If you’d like a deeper look at how to decide between SN2 and E2 pathways, along with examples, mechanisms, and practice problems, check out the post on Comparing the SN2 and E2 Reactions.
Grignard Reagent as a Carbon Nucleophile
Another very important carbon nucleophile you’ll encounter is the Grignard reagent (RMgX). It’s prepared by reacting an alkyl or aryl halide with magnesium metal in dry ether.
The carbon-magnesium bond is highly polar, making the carbon partially negative — this is the reactive nucleophilic site.
Below are the most common reactions of the Grignard reagent with aldehydes, ketones, and esters.

With aldehydes and ketones, the Grignard reagent adds once to the carbonyl carbon, forming an alcohol after the aqueous workup. With esters and acid chlorides, however, it reacts twice – the first addition forms a ketone intermediate, which then reacts again to give a tertiary alcohol.
Grignard reagents also react with other electrophiles, such as acid chlorides, epoxides, and others, and we have separate articles on these reactions linked here.
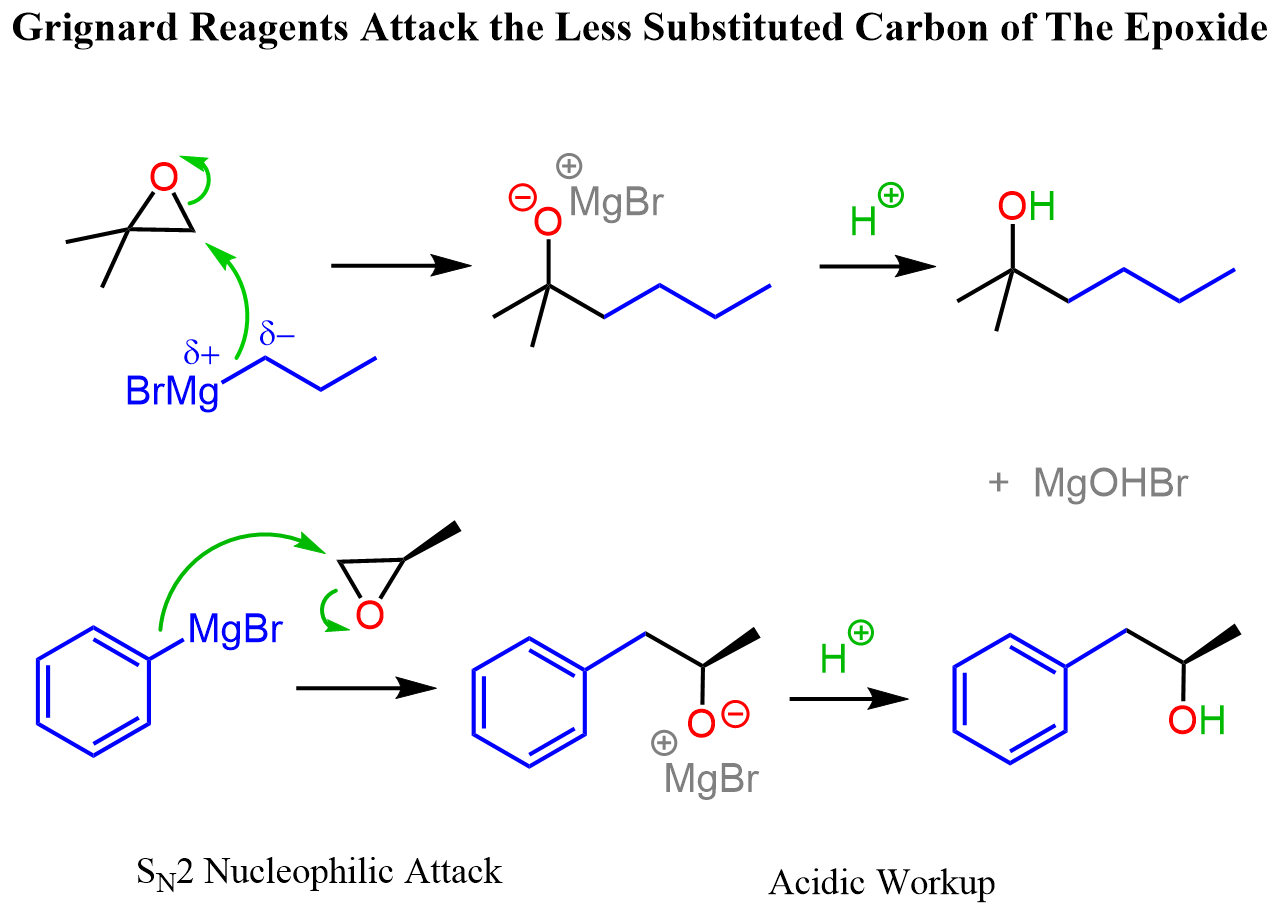
Reaction Conditions of Carbon Nucleophiles
A key point about Grignard and other organometallic reagents is that they are extremely reactive toward water, alcohols, and any other proton sources. Even moisture from the air can destroy them. That’s why they must be carried out under anhydrous (dry) conditions.
Because of this, we always show the reaction in two steps:
- The organometallic reagent reacts with the electrophile (such as a carbonyl compound).
- Then, an aqueous workup (H₂O or dilute acid) is added in a separate step to protonate the final product.
Make sure to write your reactions so the aqueous step comes after the Grignard reaction – never mix water with the reagent directly.
Organic Chemistry 2
Organolithium and Organocuprate Nucleophiles
These are going to be Organic 2 topics, so if you have not discussed them in class, you don’t need to worry about them now. In principle, these are the same as the Grignard reagent, as they are both sources of carbon nucleophiles, and they serve the purpose of extending the carbon chain.
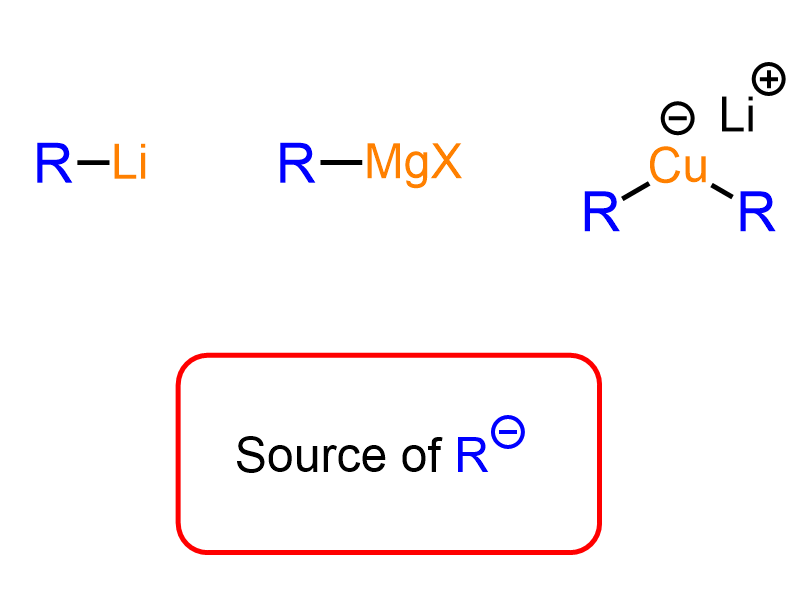
However, the difference between these organometallics is their power – how strong of a base and nucleophile they are. This depends on the polarity of the carbon-metal bond. Lithium, being the least electronegative, forms the most polar covalent bond with carbon; thus, organolithium reagents are the strongest bases and nucleophiles.
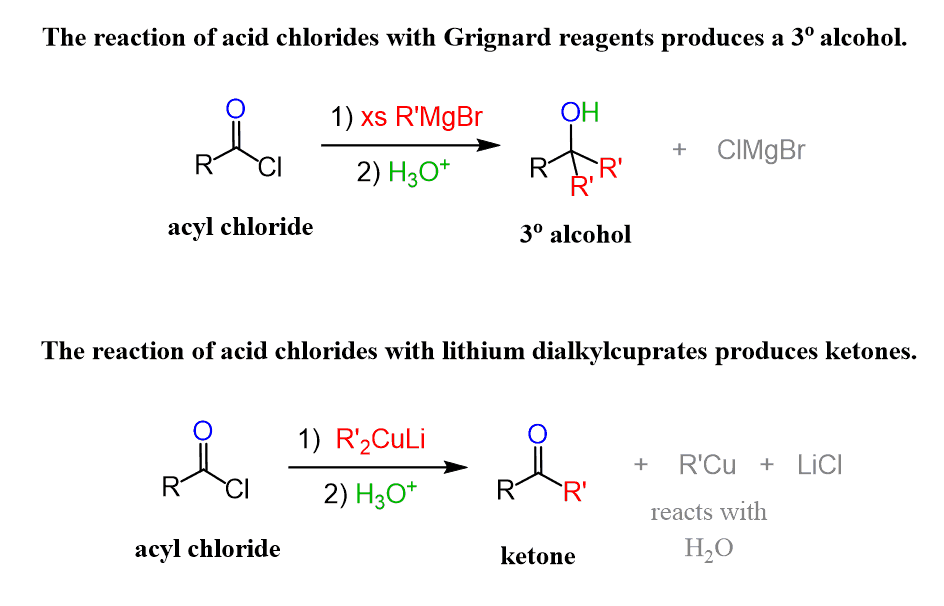
Like Grignard reagents, they tend to react with almost all derivatives of carboxylic acids, giving the same types of products. In fact, they even react with carboxylic acids because they can attack the carboxylate ion formed after the initial deprotonation of the acid.

Organocuprates, also known as the Gilman reagent, are milder and thus quite selective in who and how they react with. Among all the carbonyl-containing derivatives, they only react with acid chlorides, and unlike the Grignard reagent, they form a ketone because only one nucleophilic addition occurs.
Another important feature that sets organocuprates apart is their reaction with conjugated carbonyl compounds. Unlike Grignard and organolithium reagents, they perform a 1,4-nucleophilic addition:

We have separate posts on the reactions of Grignard, Gilman, and organolithium reagents, so check them out for more details, mechanisms, examples, and practice problems.


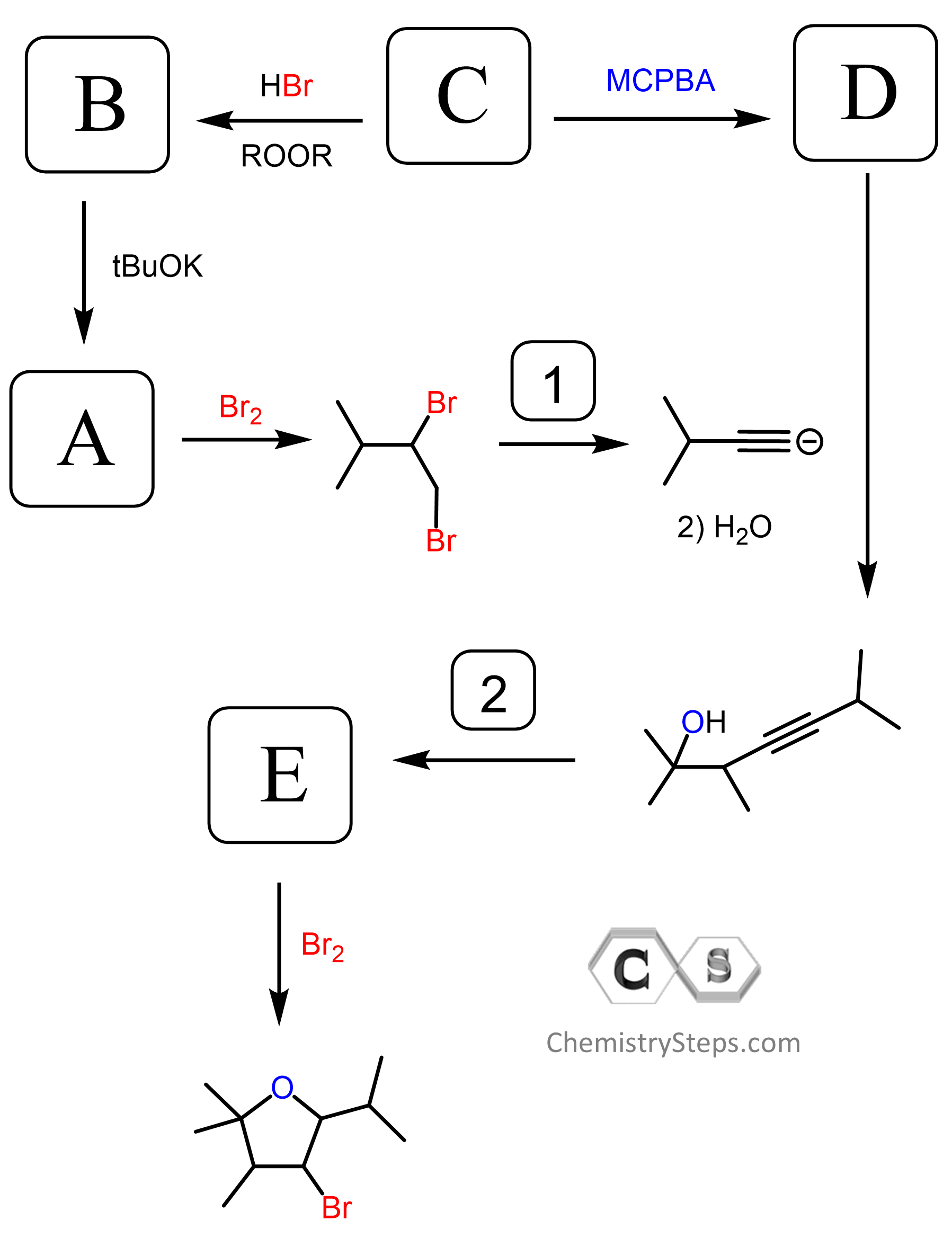
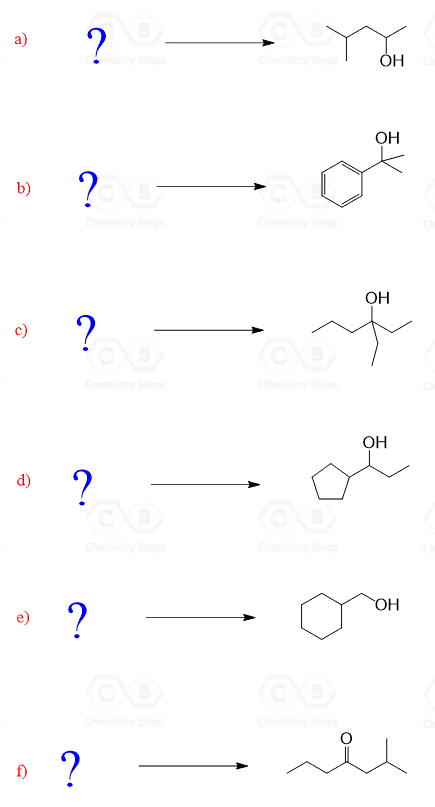
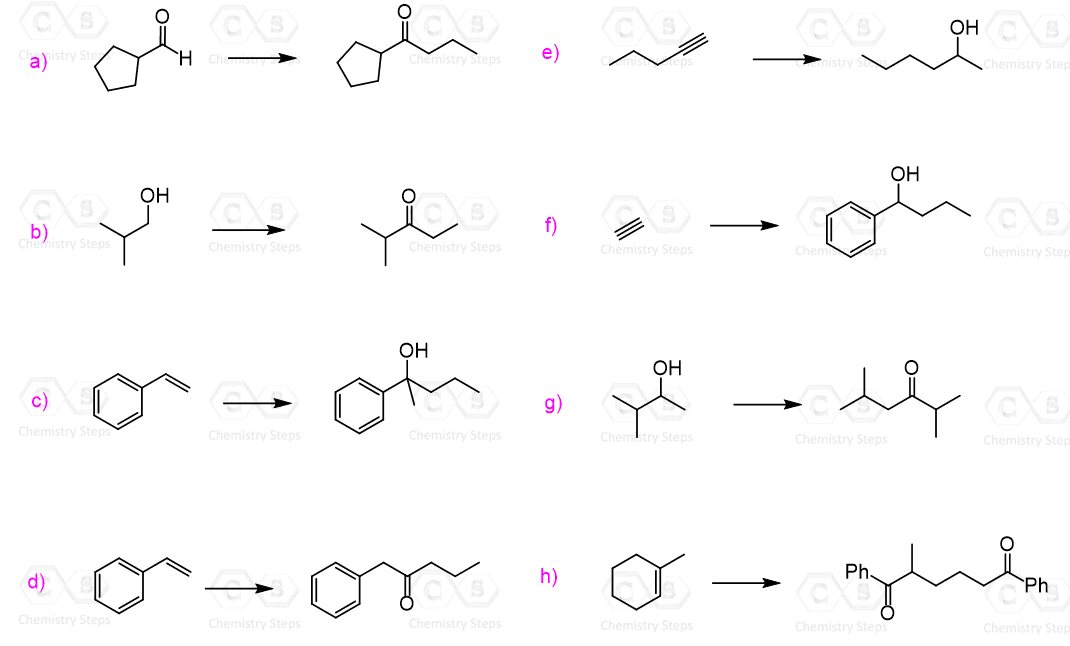
 The product has three more carbons than the starting aldehyde. Therefore, you need to think of making new C-C bonds to extend the carbon chain. So far, you have probably covered only two reactions that allow making new carbon-carbon bonds: the
The product has three more carbons than the starting aldehyde. Therefore, you need to think of making new C-C bonds to extend the carbon chain. So far, you have probably covered only two reactions that allow making new carbon-carbon bonds: the