Halogens are ortho, para directors, however, they are also deactivators!
Here is short summary to answer why halogens, being deactivators, are still ortho, para directors:

Now, let’s discuss this a bit more in detail by first doing a quick overview of the ortho, para, and meta directors.
What are Ortho-, Meta, and Para directors?
When a benzene ring with a substituent undergoes an electrophilic aromatic substitution, the electrophile is installed in a specific position(s) depending on the substituent. There are three relative positions for a disubstituted benzene ring: ortho, meta, and para.
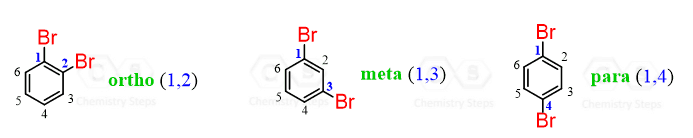
All the functional groups are divided into ortho-, para or meta-directors. To which one the group belongs depends on how it stabilizes or destabilizes the transition state of the electrophilic substitution reaction.
Activating groups (the ones that stabilize the transition state and make the reaction faster) turn out to direct the electrophile to the ortho, para positions, while the deactivating groups put them in the meta position, except for the halogens!

Halogens deactivate the aromatic ring, yet they direct the electrophile to the ortho and para positions.

But why? How does that happen, right? Well, first, a few words on the activators and deactivators.
What are activators and deactivators?
In short, the groups that donate electron density to the ring and make it electron-rich are activators. The ones that withdraw the electron density are deactivators. Activators increase the rate of electrophilic aromatic substitution and deactivators decrease it:
Two factors determine if the group is electron-donating or withdrawing. These factors are the inductive and the resonance effects. The inductive effect tells us whether the group is withdrawing the electron density from the ring because of its higher electronegativity or donating electron density to the ring, thus activating it towards electrophilic aromatic substitution. For example, alkyl groups are electron-donating by the inductive mechanism, so they make the benzene ring more reactive by pushing some electron density to it.
The resonance effect is observed when the atom connected to the aromatic ring has a lone pair(s) of electrons, which can donate electron density by the conjugated electron system, thus stabilizing the resonance contributor during the substitution reaction.

Orbital Overlap
You may wonder why we don’t take into account the fact that oxygen and nitrogen are more electronegative than carbon, and they should be pulling the electron density from the ring. This is the inductive effect, and we do count it. It is just that the resonance effect predominates here, so in other words, the oxygen and nitrogen “give” more electron density than they “take”.
For example, phenol and aniline are very reactive towards halogenation and do not require a Lewis acid like AlCl3 or FeBr3. In fact, they are so reactive that the reaction does not have any selectivity for producing a mono-halogenated product; all three 3 ortho-para positions react:

Why Are Halogens Ortho-, Para- Directors?
Like nitrogen and oxygen, the halogens F, Cl, Br, and I, are all more electronegative than carbon, and they withdraw the electron density from the ring by the inductive effect, making EAS slower than with benzene.
So, what about the resonance effect? Fluorine donates the nonbonded pair of electrons to the ring by resonance as well. However, it is so electronegative that this effect is shaded, and overall, fluorine makes the ring electron-poor and deactivates it towards electrophilic substitutions.
The other halogens, although not as electronegative, still reduce the electron density of the ring, making it less reactive in EAS. For example, the nitration of chlorobenzene is about 30 times slower than that of benzene. Had they a comparable size to the carbon, they would have a strong +M (positive mesomeric, resonance donating) effect, making the EAS faster than those with benzene. However, they are all significantly larger, and so are their orbitals bearing the lone pairs, and thus they do not show a strong resonance effect either. Once again, the reason for this is the poor overlap between the 2p orbital of the carbon and the 4p and 5p orbitals of chlorine and bromine. Remember, the conjugation and bond-making depend on the efficiency of the orbital overlapping.
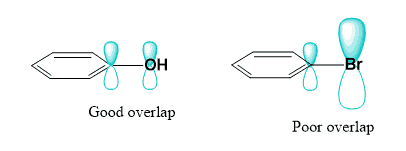
Orbital overlap
Now, as to why they are ortho-, para- directors, you need to remember that they are still electron-donors by resonance, which does stabilize the transition state by an additional resonance contributor, whether it is significant or not.

To keep this in a nutshell, remember that halogens deactivate the ring, but they still orient the substitution to the ortho and para positions because of the resonance effect.
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds

You’ve been very helpful! Thank you!
Good to hear that!
Halogens are deactivating but ortho and para directing ,They increase acidic strength or decrease acidic strength ???
Increase acidic
Why activators direct incoming electophiles to ortho and para position only while deactivators direct to meta position?
For that, you need to learn the principles of ortho, meta, para substitutions:
Ortho Para Meta in EAS
Halogens are deactivate but ortho and para director because of their high electronegativity.
Thanks so much
You are welcome!