The Diels-Alder reaction is very important in Organic Chemistry. In fact, Otto Diels and Kurt Alder received the 1950’s Chemistry Nobel Prize for the discovery of this reaction back in 1928. You will see it quite often in organic synthesis problems as it is a unique way of making cyclic structures from acyclic reactants.
For example, let’s work on this practice problem:
Show how to synthesize the following compound from cyclohexane:
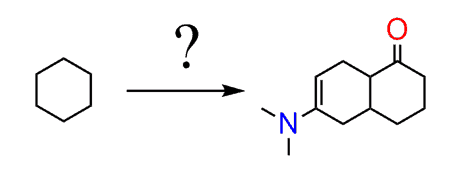
Suppose you are given this problem on a test and don’t necessarily know that it involves a Diels-Alder reaction.
Let me give you one hint: whenever you see a six-membered ring with a double bond, think about a Diels-Alder reaction!
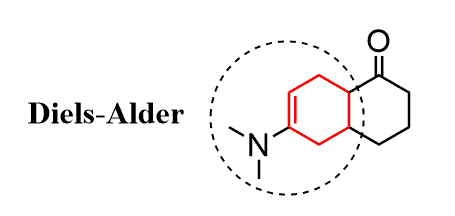
This ring system would have come from the reaction of the following diene and the dienophile (check this post on determining the starting material of Diels-Alder reaction):
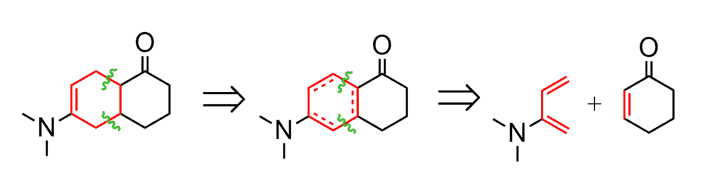
So, we have our starting material cyclohexane turned into an ɑ, β-unsaturated carbonyl:
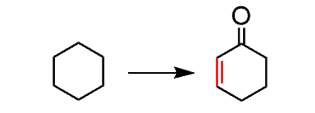
This means, we first need to incorporate a double bond. The only way of introducing a functional group to an alkane is the radical halogenation. For selective halogenation, Br2 must be used, however, since cyclohexane is symmetrical, you can use Cl2 as well:
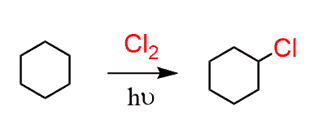
Next, treat this alkyl halide with a strong base. We can use NaOH and that would work fine, however to reduce the percentage of substitution reaction, you can also use a sterically hindered base such as potassium tert-butoxide (tBuOK):
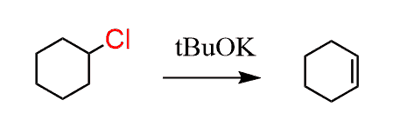
Now, for the carbonyl; notice that it is in the allylic position (next to the double bond).

How do you functionalize the allylic position?
If you recalled the allylic bromination, then great, you can now think about how to convert the bromide into a carbonyl.
As a hint, think about an SN2 and oxidation reactions.
So, this can be achieved in two steps; first, convert the chloride into an alcohol by reacting with NaOH:
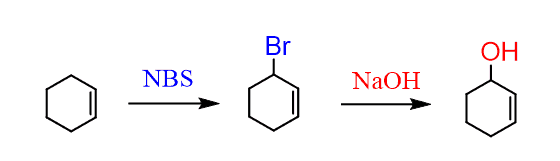
Step 2; oxidize the alcohol to a carbonyl. And since it is a secondary allylic alcohol, it can be oxidized with a mild oxidizing agent. Even strong oxidizing agents would be fine but generally, you want to be considerate when using strong reagents. So, you can use, PCC or MnO2 for example:
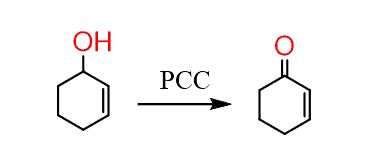
At this point, we have prepared the dienophile which needs to be reacted with the corresponding diene.
There is a nitrogen on the diene which means you also need to pay attention to the regioselectivity of this Diels-Alder.

You have two ways to determine the proper alignment of the diene and the dienophile.
One is to draw the resonance structures of the diene and dienophile placing the formal charges on the terminal atoms:
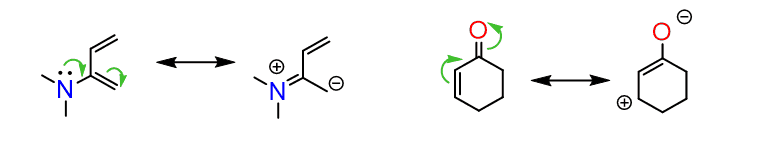
The molecules must align such that the positive and negative charges are next to each other:
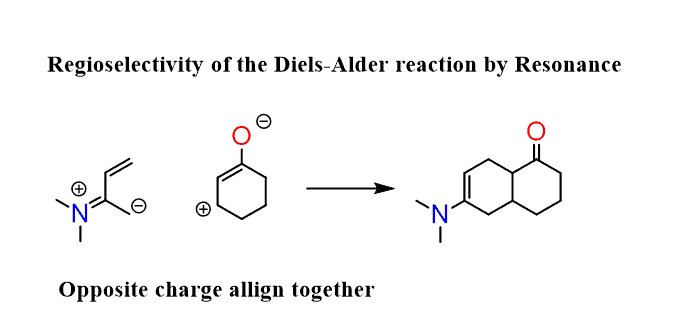
You can also do this without drawing the resonance forms. Simply place the molecules next to each other and draw the curved arrows you’d use for resonance structures:
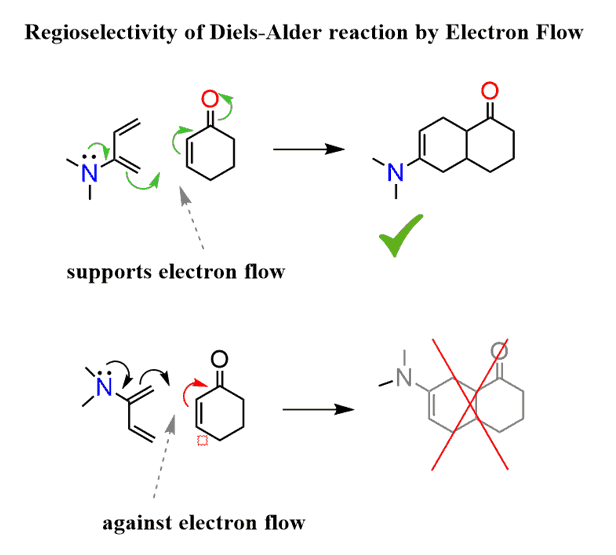
The correct alignment is the one that supports the flow of electron from the electron-donating diene substituent to the electron-withdrawing group of the dienophile.
What do you think about the stereochemistry of this reaction? The starting materials are achiral; therefore, a racemic mixture of enantiomers is obtained.

It is not always the case that achiral reactants form a racemic mixture, but it is beyond the scope of this problem.
In summary, here how you can synthesize the target product from cyclohexane:

Below are some multistep synthesis practice problems and each of these, besides everything else, involves a Diels-Alder reaction at one point.
If you need to polish your skills in the main aspects of the Diels-Alder reaction, you can try to work on these practice problems first:
- Predict the Products of the Diels-Alder Reaction with Practice Problems
- Endo and Exo products of Diels-Alder Reaction with Practice Problems
- Regioselectivity of the Diels–Alder Reaction with Practice Problems
- Identify the Diene and Dienophile of the Diels–Alder Reaction with Practice Problems








Professor: In Synthesis #3, would you have any of the HCl add across the double bond and make a secondary chloro-compound?
Off the top of my head, I would say Zn/H+ should not reduce or react with an isolated C=C bond, but it is a valid concern. A better option would perhaps be the Wolff–Kishner reduction which is done under basic conditions.