The amine functional group is one of the most common and important nitrogen-containing groups in organic chemistry. Amines are derivatives of ammonia (NH₃), where one or more hydrogen atoms are replaced by alkyl or aryl groups.
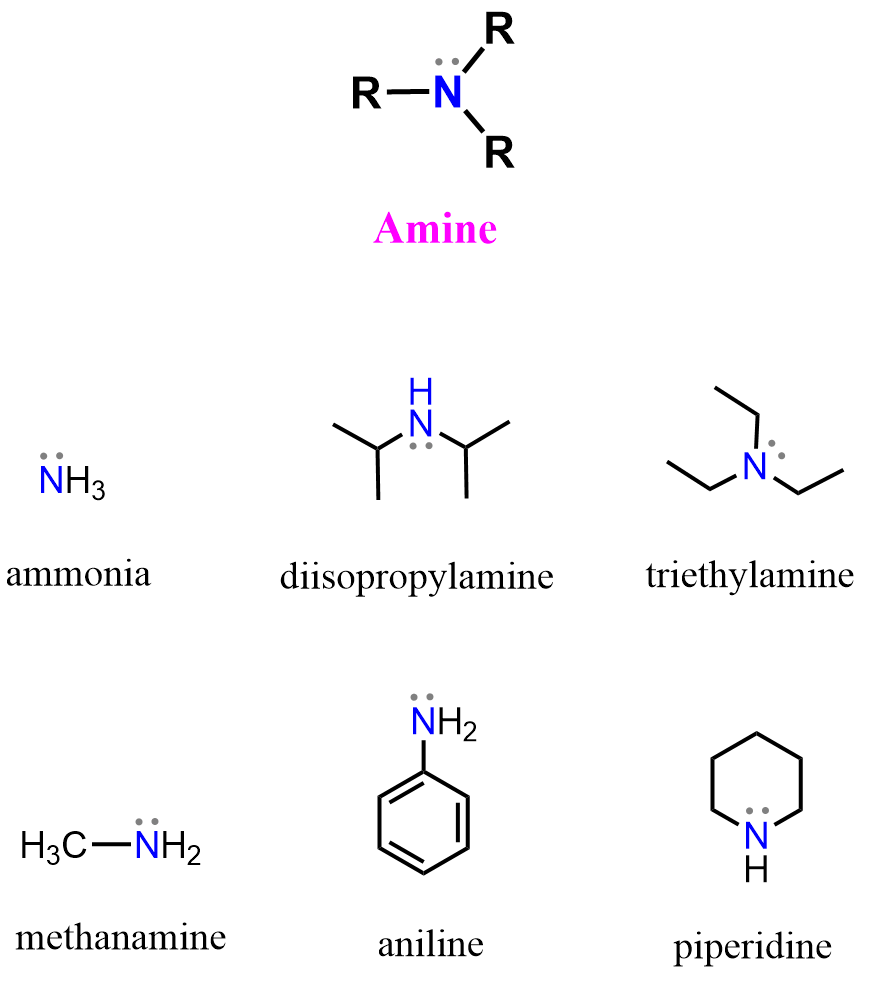
Amines play central roles in biological systems, pharmaceuticals, dyes, and polymers, and are essential intermediates in many organic reactions.
Structure of Amines
Amines contain a nitrogen atom bonded to one, two, or three carbon-containing groups (alkyl or aryl). Depending on the number of carbon groups attached to the nitrogen, amines are classified as:

- Primary amine (1°): one carbon group attached → R–NH₂
- Secondary amine (2°): two carbon groups attached → R₂NH
- Tertiary amine (3°): three carbon groups attached → R₃N
Classification of Amines
If the nitrogen is part of a ring, the compound is called a cyclic amine (for example, pyridine, pyrrole, pyrrolidine, and piperidine etc).
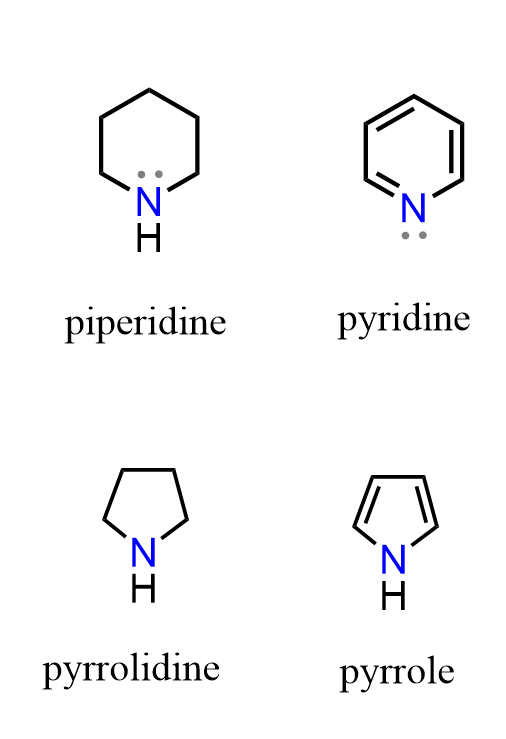
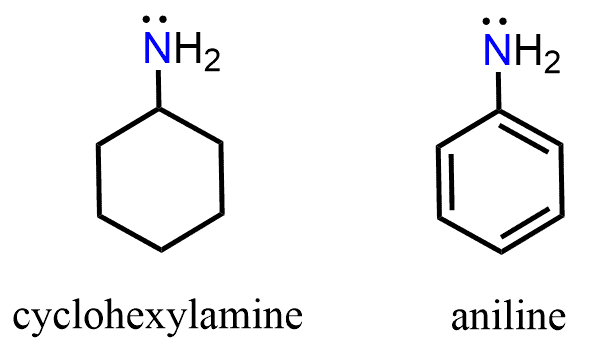
Aromatic amines, such as aniline, have the nitrogen directly bonded to an aromatic ring, which affects their basicity due to resonance between the nitrogen’s lone pair and the aromatic π system.
Geometry and Hybridization in Amines
The nitrogen atom in amines is generally sp3 hybridized, forming a trigonal pyramidal geometry.
This geometry arises because nitrogen forms three sigma bonds and keeps one lone pair. The lone pair pushes the bonds slightly closer together, giving bond angles around 107°, smaller than the 109.5° in a perfect tetrahedron.
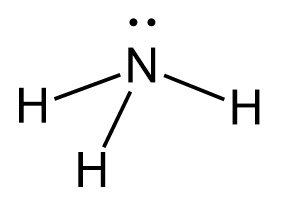
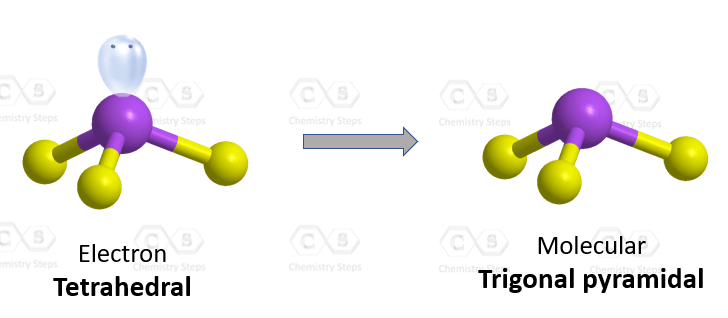
Let’s demonstrate this with the example of ammonia (NH₃), although the same principle applies to any amine, regardless of the size of the molecule. In ammonia, the nitrogen atom is bonded to three hydrogen atoms and has one lone pair of electrons.
- The electron geometry considers all regions of electron density – the three N–H bonds plus the lone pair. This gives a tetrahedral electron geometry with a steric number of 4.
- The molecular geometry considers only the positions of the atoms (ignoring lone pairs), resulting in a trigonal pyramidal shape.
You can read more about steric numbers, electron, and molecular geometries in our VSEPR post.
The nitrogen in amines can adopt either sp3 or sp2 hybridization, depending on its bonding environment. For example, in typical aliphatic amines, where nitrogen forms only single bonds, it is sp3 hybridized with a trigonal pyramidal geometry.
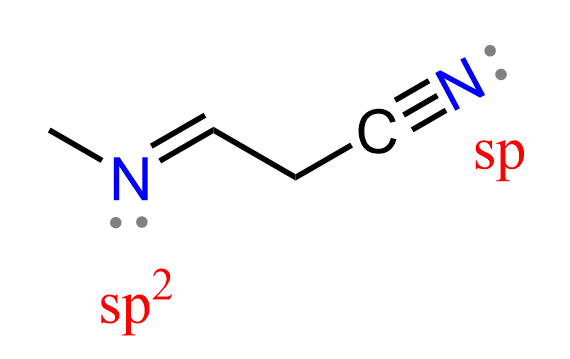
To be more accurate, the double-bonded and triple-bonded nitrenes are not amines but rather imines and nitriles, and I have only added them to demonstrate examples of nitrogen being sp2 and sp hybridized.
A notable exception occurs when a nitrogen with only single bonds is adjacent to a π bond. In this case, the lone pair on nitrogen delocalizes over the aromatic π system, which is only possible if the nitrogen adopts sp2 hybridization.
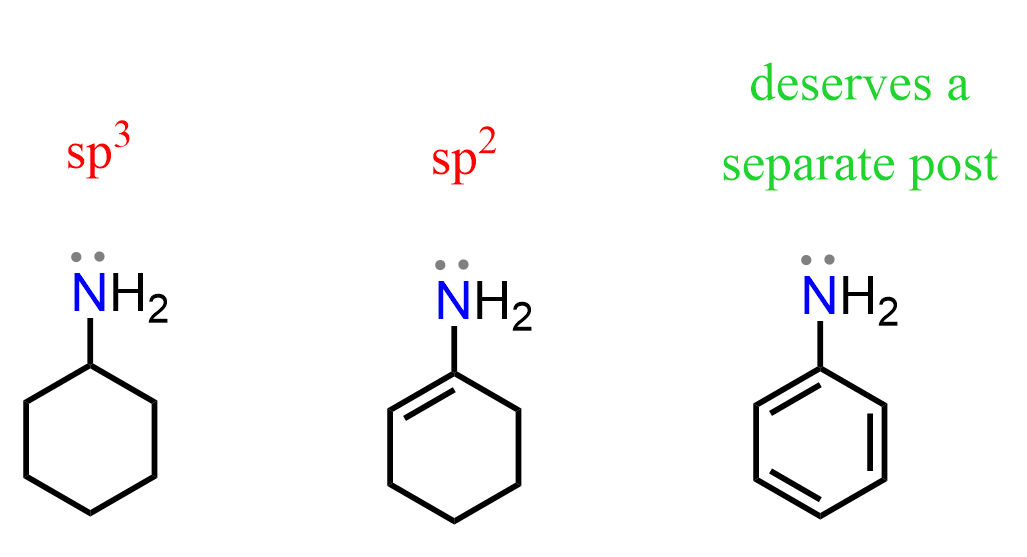
This delocalization reduces the availability of the lone pair for protonation, which explains why aromatic amines are less basic than aliphatic amines.
The Nomenclature of Amines
In general, amines can be named either by systematic or common names.
There are lots of amines that are simply referred to by their common names. Most of these are used as organic bases and constitute a part of biologically active and essential compounds such as amino acids, nitrogen-containing pain killers (alkaloids), as well as synthetic medicines.

Systematic Nomenclature of Amines
Naming amines by the systematic nomenclature follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes.
This is the brief summary of naming a primary amine:
Step 1. Identify the longest carbon chain bonded to the amine nitrogen.
Step 2. Identify the substituents.
Step 3. Number the parent chain, giving the amine the lowest locant
Step 4. Put everything together, having the substituents in alphabetical order.
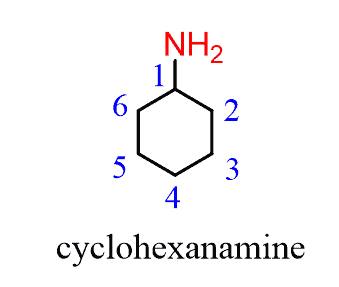
For example, butane changes to butan-1-amine, cyclohexane to cyclohexanamine:

In common names, we treat the carbon chain as an alkyl group bonded to the nitrogen atom. The alkyl group is added to the suffix “amine,” forming a single word:
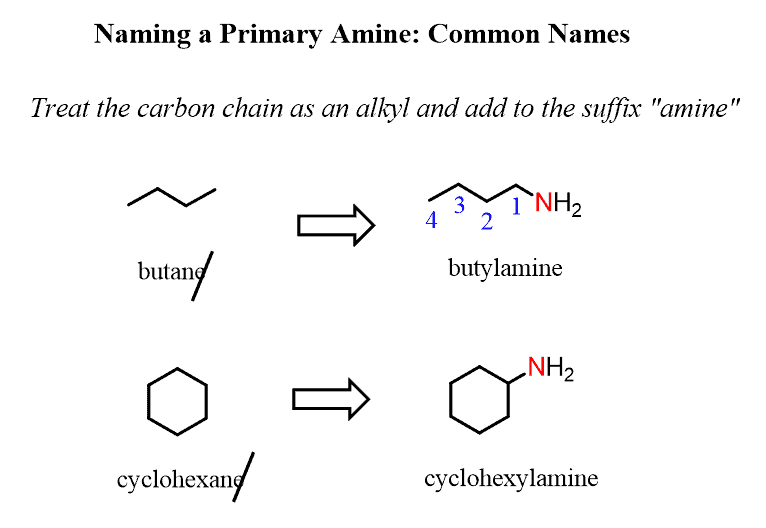
Notice that when the amine is connected to a ring, we start numbering from the carbon connected to the NH2 group. This rule always puts the NH2 group at C1; therefore, the “1” is usually omitted from the name:
We have a separate post dedicated to the nomenclature of amines, so check it out for more details, examples, and practice problems.
Physical Properties of Amines
Boiling Point of Amines
Amines generally have higher boiling points than alkanes but lower than alcohols. This is because the lone pair on nitrogen allows amines to engage in hydrogen bonding, an important type of intermolecular interaction. Hydrogen bonding increases the attractive forces between molecules, requiring more energy (higher temperature) to separate them during boiling.
Primary and secondary amines can form stronger hydrogen bonds due to the presence of N–H bonds, while tertiary amines lack N–H bonds and therefore have weaker intermolecular interactions.

Notice that the boiling point increases as we go from the tertiary to the primary amine as a result of increasing the number of hydrogen bonds per nitrogen. Propylamine has two hydrogens connected to the nitrogen, and each can make a hydrogen bond with a neighboring nitrogen atom from another molecule.
You can read more about these interactions in our Hydrogen Bonding and Intermolecular Forces post.
The hydrogen bonding ability of amines plays a crucial role in biology. For example, in DNA, the nitrogen atoms in aromatic rings known as nucleobases or nitrogenous bases act as basic sites capable of forming hydrogen bonds. These nucleobases (adenine, thymine, guanine, and cytosine) pair through complementary hydrogen bonding (A:T and G:C), allowing genetic information to be stored and transferred.

We will discuss the basicity of amines below to clarify why we call them nucleobases.
Solubility of Amines
Small amines are soluble in water because they can form hydrogen bonds with water molecules. As the alkyl chain length increases, the hydrophobic carbon portion becomes larger, reducing solubility despite the polar NH₂ group. This balance between polar and nonpolar interactions explains why short-chain amines are more water-soluble, while long-chain amines behave more like hydrocarbons.
The Odor of Amines
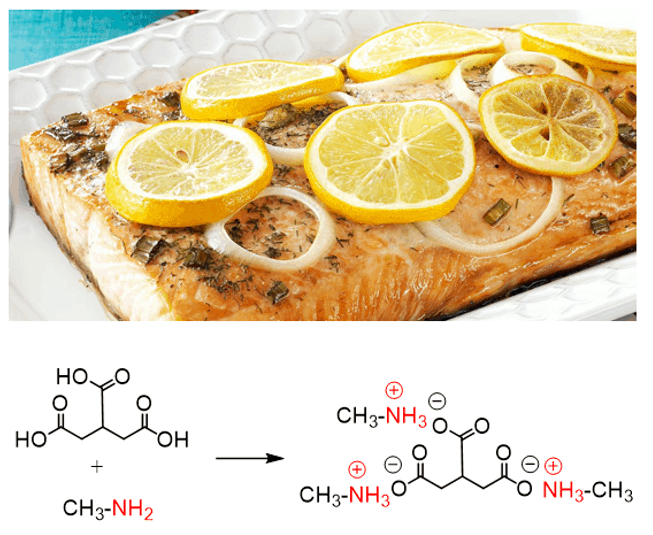
Many amines have strong, fishy, or ammonia-like smells, especially low-molecular-weight ones. Because amines are organic bases, lemon juice is often added when cooking fish to neutralize the fishy odor. The citric acid in lemon reacts with the amines produced during fish decomposition, forming non-volatile ammonium salts, which eliminates the characteristic fishy smell.
Preparation of Amines
Amines can be prepared by several methods, including substitution and reduction reactions. Some of the common ways of preparing amines are shown below,
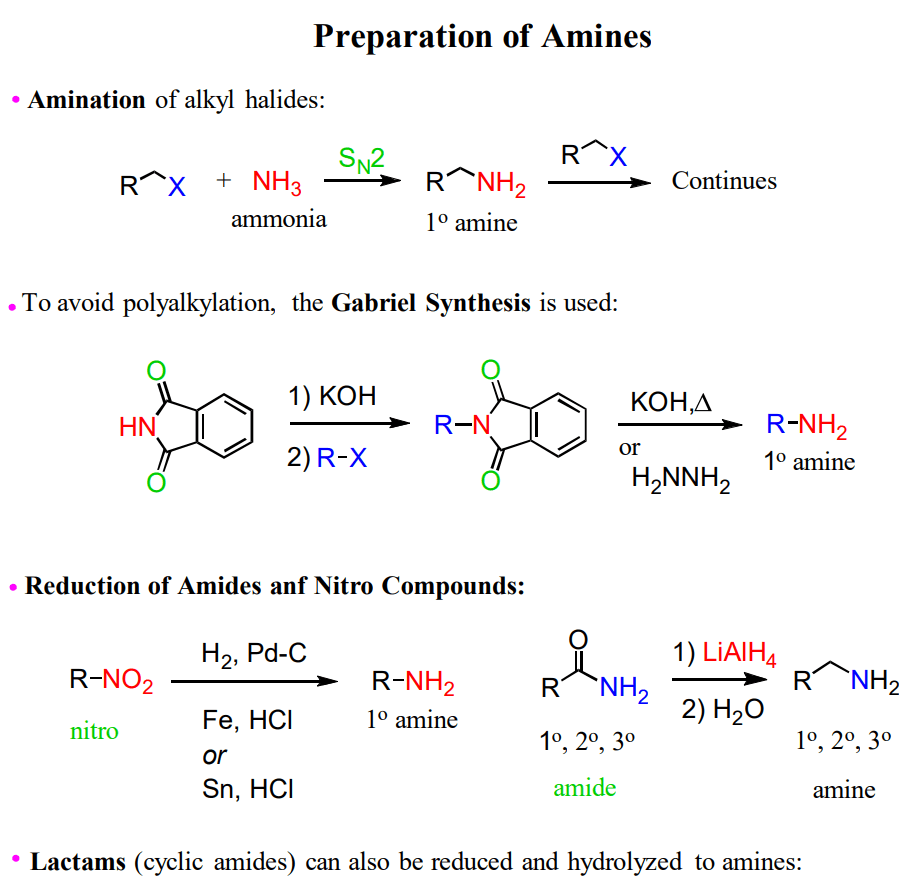
You can also check this post for more reactions and mechanisms, such as Reductive Amination of Aldehydes or Ketones and Gabriel Synthesis
Basicity of Amines
Although amines are considered weak bases, they are still the most basic sites in neutral organic molecules because nitrogen’s lone pair makes them stronger bases and nucleophiles than most other neutral compounds.
So, what makes amines basic? According to the Brønsted–Lowry theory, bases are proton acceptors, while in the Lewis theory, they are electron pair donors. The lone pair on the nitrogen atom allows amines to act as both, readily accepting a proton to form an ammonium ion (RNH₃⁺) when mixed with an acid.
As an example, consider the acid-base reaction between ethyl amines and hydrochloric acid:

Ethylamine, like other amines, has a lone pair of electrons on nitrogen that can accept a proton (H⁺) from the acid. When this happens, ethylammonium chloride is formed – an ammonium salt where the nitrogen is now positively charged (–NH₃⁺) and paired with a chloride anion (Cl⁻). This reaction illustrates the basic character of amines and how they form stable salts when treated with acids.
We have a separate post on the basicity of amines, which discusses their base strength, factors affecting basicity, and comparative trends in detail.
Reactivity of Amines
We have seen in a simple reaction between ethyl amine and HCl how amines act as bases. Here are two more examples of amines acting as organic bases and abstracting protons with their lone pair:

The presence of the lone pair on the nitrogen makes them also nucleophilic, and they participate in nucleophilic substitution reactions. For example, primary amines react readily with primary alkyl halides, forming secondary amines.
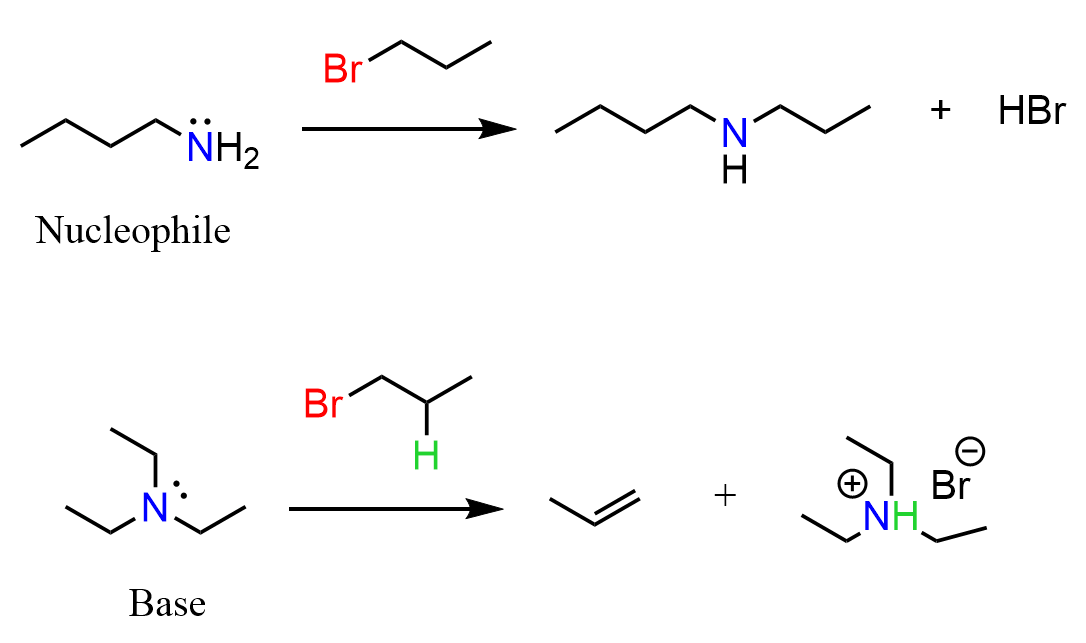
As the number of substitutions increases on the nitrogen, the basic properties predominate because large (bulky) species participate in elimination reactions rather than substitution reactions. The nucleophilic substitution and elimination reactions are covered later in Organic Chemistry 1, once all the functional groups and other topics explaining the structure of organic molecules are covered.
Amines in Nature and Medicine
Amines are incredibly important in biology and medicine because the nitrogen atom’s lone pair allows them to form hydrogen bonds, accept protons, and interact with receptors in the body. This property makes them essential in neurotransmitters, natural products, and countless pharmaceuticals.
🧬 Alkaloids and Natural Amines
A large class of naturally occurring amines, known as alkaloids, includes many biologically active compounds such as:
- Nicotine – a tertiary amine from tobacco that stimulates the nervous system by binding to nicotinic receptors.
- Caffeine – part of a heterocyclic polyamine structure that acts as a central nervous system stimulant.
- Morphine and Codeine – tertiary amine alkaloids from the opium poppy used as powerful painkillers.
- Cocaine – a tertiary amine that functions as a stimulant and local anesthetic (though highly addictive).
The amino group in these molecules is what gives alkaloids their basicity, solubility in acids, and strong physiological effects on the nervous system.
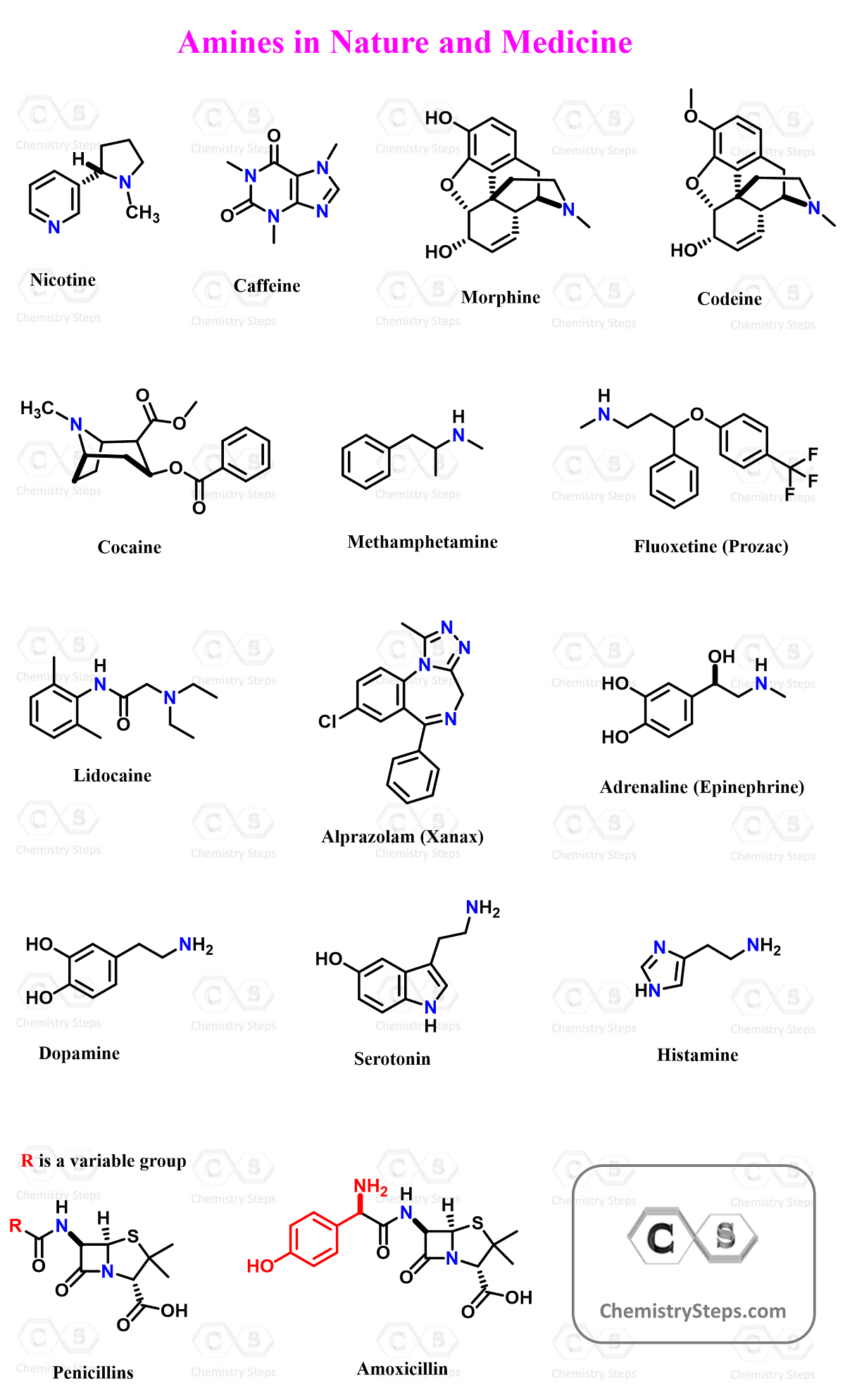
💊 Pharmaceutical Amines
Many important drugs also contain amine groups:
- Amphetamine and Methamphetamine – primary amines that act as stimulants by enhancing neurotransmitter activity.
- Prozac (Fluoxetine) – a secondary amine antidepressant that increases serotonin levels.
- Xanax (Alprazolam) – a triazolobenzodiazepine containing a nitrogen atom that acts on the central nervous system to reduce anxiety.
- Lidocaine – a tertiary amine used as a local anesthetic.
- Epinephrine (Adrenaline) – a secondary amine hormone that triggers the “fight-or-flight” response.
- Penicillin and Amoxicillin – antibiotics that contain both amine and amide groups, essential for their activity.
🧠 Biogenic Amines (Neurotransmitters)
Naturally occurring amines such as dopamine, serotonin, and histamine act as neurotransmitters, regulating mood, alertness, and immune responses.
So, yes, amines are everywhere, from the caffeine in your morning coffee to painkillers, antibiotics, and antidepressants.
Summary of Amines
The amine functional group is a fundamental building block in both organic and biological chemistry. The lone pair of electrons on the nitrogen atom makes amines basic, nucleophilic, and highly versatile, allowing them to participate in a wide range of reactions.
Amines are classified as primary (one carbon group attached to nitrogen, R–NH₂), secondary (two carbon groups attached, R₂NH), or tertiary (three carbon groups attached, R₃N). Understanding this classification, along with their structure and reactivity, provides a foundation for studying related biologically and medicinally important molecules.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
- Boc Protecting Group for Amines
