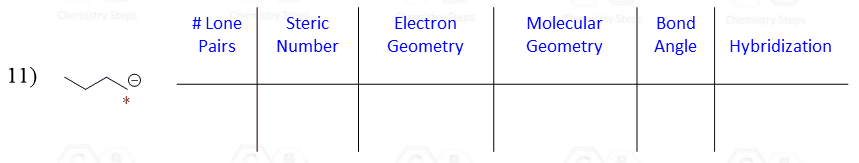
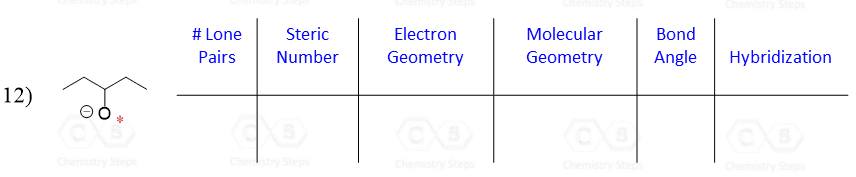
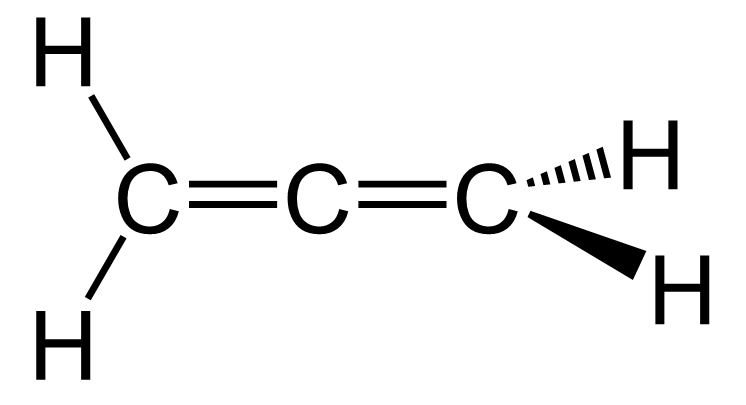
The hybridization theory is often seen as a long and confusing concept, and it is a handy skill to be able to quickly determine if the atom is sp3, sp2, or sp without having to go through all the details of how the hybridization happened.
Fortunately, there is a shortcut in doing this, and in this post, I will try to summarize this in a few distinct steps that you need to follow.
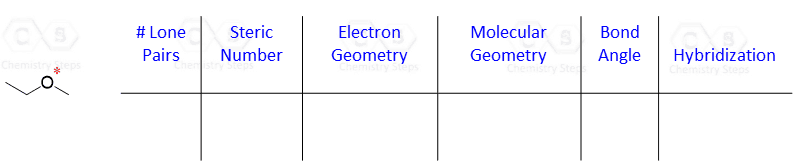
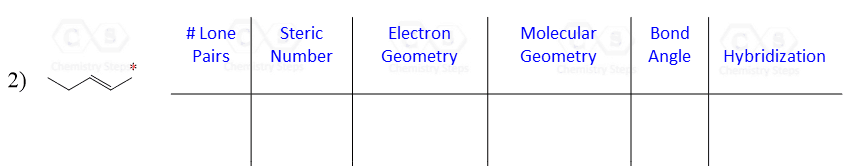
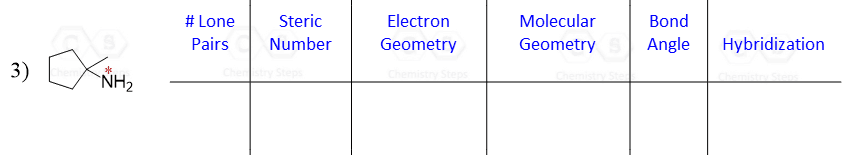
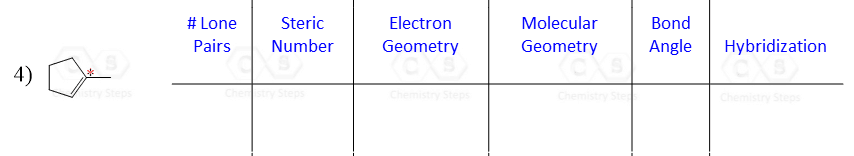
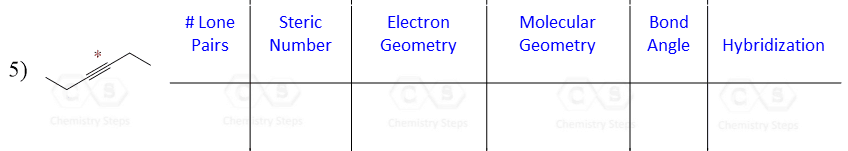
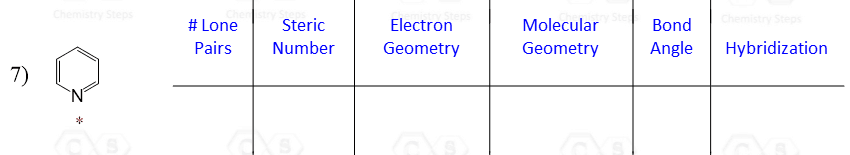
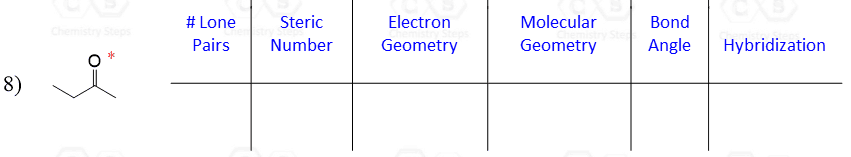
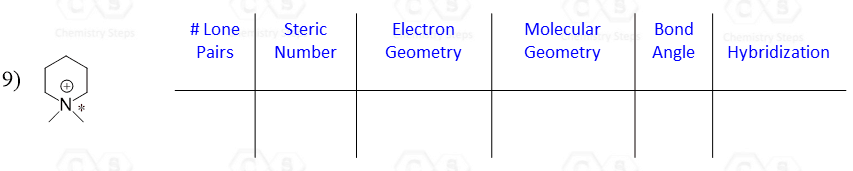
Let’s say you are asked to determine the hybridization state for the numbered atoms in the following molecule:

The first thing you need to do is determine the number of groups that are on each atom. By groups, we mean either atoms or lone pairs of electrons. This is also known as the Steric Number (SN).

Below are a few examples of steric numbers 2-4, which are largely what you need to know in organic chemistry:

Notice that multiple bonds do not matter if it is atoms + lone pairs for any bond type.
Once you know how to determine the steric number (it is from the VSEPR theory), you simply need to apply the following correlation:
If the steric number is 4, it is sp3
If the steric number is 3 – sp2
If the steric number is 2 – sp
So now, let’s go back to our molecule and determine the hybridization states for all the atoms.
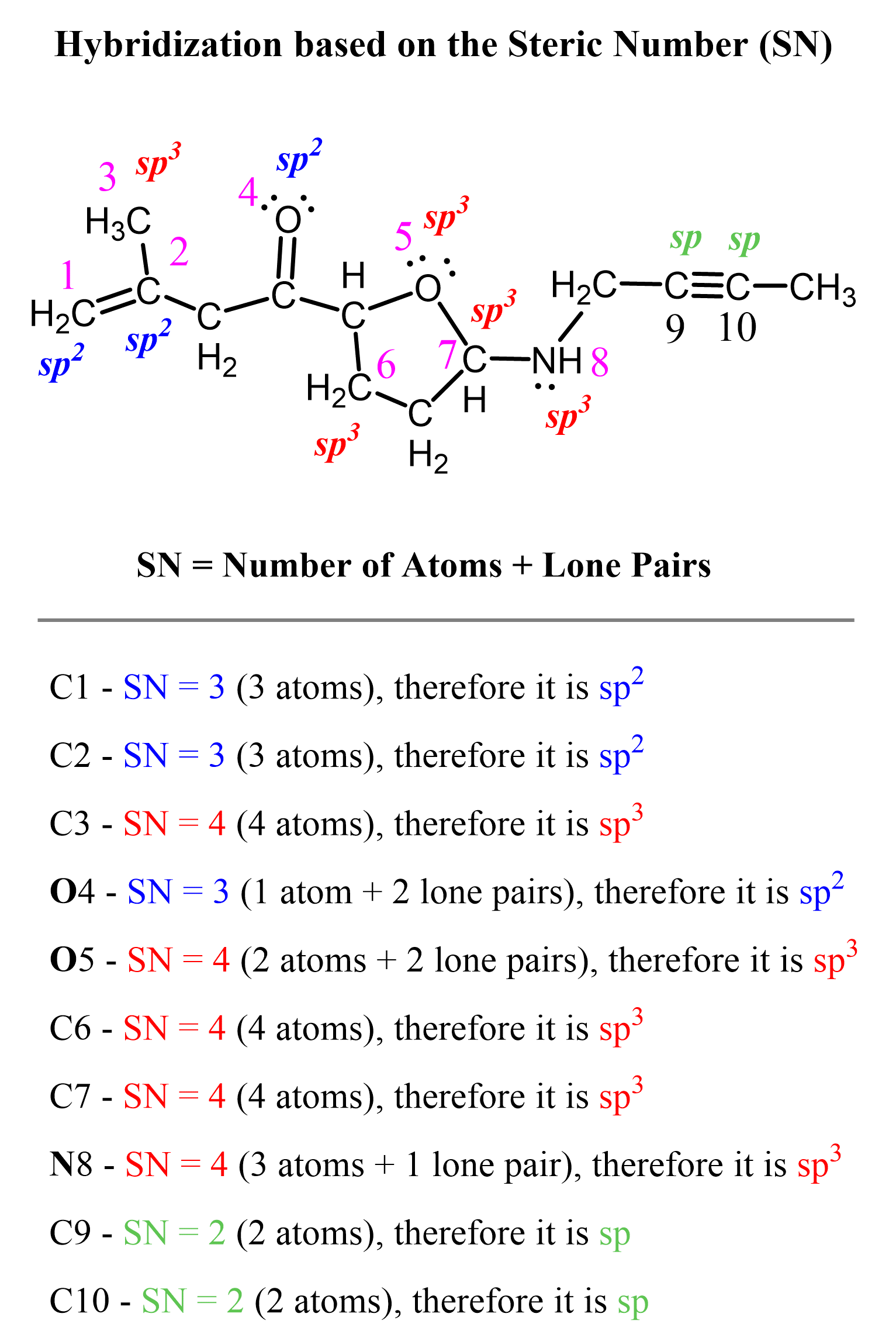
Other methods to determine the hybridization
In addition to this method, it is also very useful to remember some traits related to the structure and hybridization. In general, an atom with all single bonds is sp3 hybridized. The best example is the alkanes. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry.
The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have trigonal planar geometry.
The triple bond, on the other hand, is characteristic of alkynes, where the carbon atoms are sp-hybridized.
Some exceptions
There are a few common exceptions to what we have discussed about determining the hybridization state, and they are mostly related to the method by which we look at the bonding type of the atom.
For example, in carbon dioxide (CO2), the carbon has two double bonds, but it is sp-hybridized.
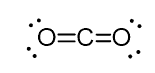
And the reason for this is the fact that the steric number of the carbon is two (there are only two atoms of oxygen connected to it), and in order to keep two atoms at 180o, which is the optimal geometry, the carbon needs to use two identical orbitals. This is only possible in the sp hybridization. The other two 2p orbitals are used for making the double bonds on each side of the carbon.
Another common, and very important example is the carbocations.

Here, the carbon has only single bonds, and it may look like it is supposed to be sp3 hybridized. However, the carbon in this type of carbocation is sp2 hybridized. Again, for the same reason, its steric number is 3 (sp2 – three identical orbitals).
An exception to the Steric Number method
One exception with the steric number is, for example, the amides. The nitrogen atom here has a steric number of 4 and is expected to be sp3. However, because of the resonance delocalization of the lone pair, it interconverts from sp3 to sp2 as it is the only way of having the electrons in an aligned p orbital that can overlap and participate in resonance stabilization with the pi bond electrons of the C=O double bond.

We have a separate post dedicated to the effect of lone pairs on the hybridization, so check that out for such examples.