In the previous post, we talked about Frost circles which are a great tool in assessing the aromaticity of cyclic conjugated systems. Now, the largest conjugated cycle we got to was cyclooctatetraene which, based on Huckel’s rule and Frost circle is expected to be aromatic. However, because of the ring strain, it does not adopt a planar enough geometry to support the needed conjugation of the orbitals (Chemical Reviews, 2006, Vol. 106, No. 12 5347):

A similar situation is also observed with cyclooctatetraene, which, having 4n electron, is predicted to be antiaromatic, but once again, because of its nonlinear geometry, is nonaromatic:

So, a good question here is what about even larger cyclic systems? Can we still use Huckel’s rule and apply the 4n + 2 electron count to distinguish aromatic and antiaromatic compounds? Perhaps, the person who was most curious about this, and succeeded in preparing higher cyclic conjugated polyenes was Professor Franz Sondheimer at University College in London. He synthesized a series of large cyclic molecules with fully conjugated double bonds. To avoid complicated nomenclature, he classified them and any cyclic conjugated hydrocarbons as Annulenes. For example, [14]annulene, [16]annulene, [18]annulene, and we can use [6]annulene for benzene, [4]annulene for cyclobutadiene, and [8]annulene for cyclobutadiene. The number of carbon atoms in the ring is indicated first.

[12]Annulene
The next ring in the series is [12]annulene, which has 12 π electrons, and based on Huckel’s rule is expected to be antiaromatic. Indeed, it was found to exhibit antiaromatic properties therefore, it must have a sufficiently planar geometry. Remember, we mentioned in earlier posts that if the molecule can avoid being antiaromatic, it will do it by distorting the planar structure. So, you may wonder how come [12]annulene doesn’t. One may explain this by the energy balance that the molecule tends to find. On one hand, it wants to avoid being antiaromatic, but on the other hand, adopting certain geometries or avoiding them comes with a cost due to unfavorable bond angles, ring strain, steric effects, etc. So, apparently, it is energetically more favorable for [12]annulene to be antiaromatic but keep the nearly planar and perfect 120o bond angles than twisting them to be nonplanar.
It is worth mentioning that [12]Annulene is quite unstable, and there are challenges in isolating and studying it. It undergoes a cis-trans isomerization, which may also have an impact on how nonaromatic or antiaromatic it is.
In general [12]Annulenes are a complex subject to draw a clear borderline of nonaromatic and antiaromatic characteristics. I personally accept either of the two answers from students, provided a good explanation is given.
You can check this article “The [12]Annulene Global Minimum” (Org. Chem. 2008, 73, 4, 1532–1535) and those at the end of the text for more information on [12]Annulenes.
[14]Annulene
Like [12]annulene, [14]annulene also keeps its planar geometry, but this time it benefits more from that because aromaticity is achieved.
In general, the trend is that as long as an annulene with 4n + 2 delocalized electrons is nearly planar, it will be aromatic, whereas those with 4n electrons are antiaromatic. If it is not planar, the annulene is nonaromatic. This shouldn’t be news at this point anyway, but I just wanted to mention it and emphasize that we explain the experimental data and not just rely on different theories.
So, the next question would be what experimental data we use to distinguish aromatic, nonaromatic, and antiaromatic compounds.
Distinguishing Aromatic, Nonaromatic, and Antiaromatic Molecules
One of the experimental indicators is the unusual; stability of aromatic compounds that we have discussed earlier. For example, how benzene and cyclooctatetraene react differently with bromine.


An interesting feature of aromatic compounds is the characteristic high ppm values in NMR spectroscopy compared to regular alkenes. Aromatic protons appear in the 7-8 ppm region while regular alkenes give a signal in the 4-5 ppm region.
This is due to a phenomenon called magnetic anisotropy. When protons on a carbon-carbon double bond are placed in a magnetic field, the circulating π electrons create a local magnetic field that adds to the applied field which causes them to experience a stronger net field and therefore resonate at a higher frequency:

This effect is more pronounced in aromatic compounds, which have resonance in the range from 7 to 8 ppm. The circulation of the p electrons in benzene is called a ring curren,t and the protons experience an additional magnetic field that is induced by this ring current.
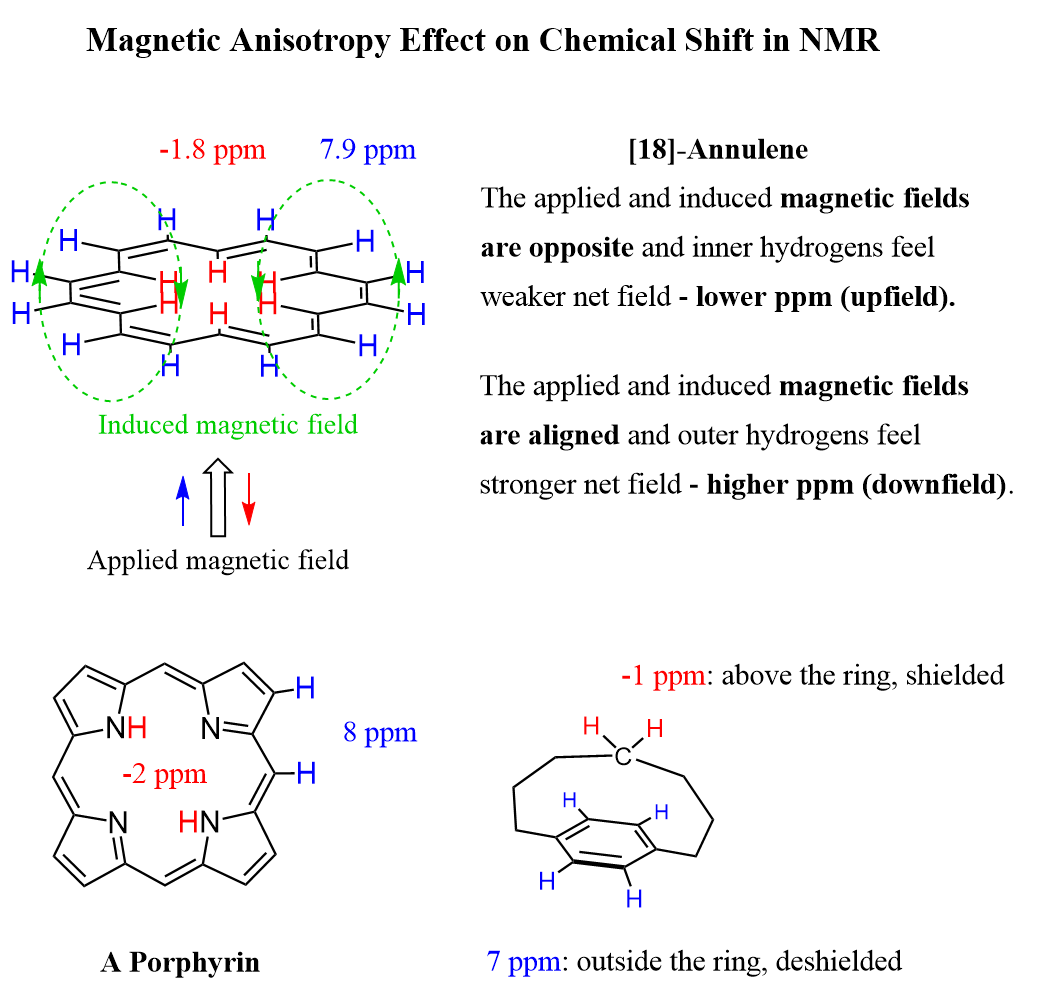
What makes larger annulenes unique in this regard is that they have protons both inside and outside of the ring. For example, let’s consider [18]annulene, which exhibits aromatic properties:
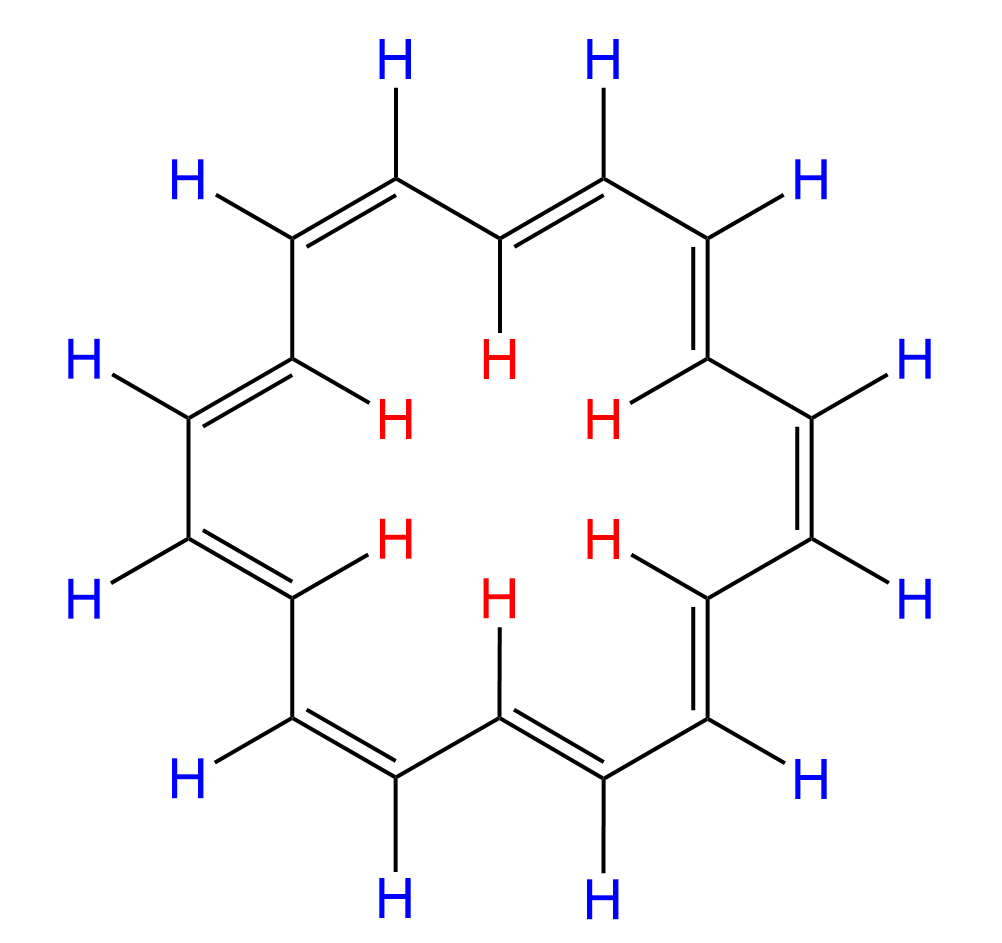
Now, why is the presence of protons in the inner and outer areas of the ring important?
To answer this question, notice in the image above that in the inner area of benzene, the induced magnetic field is aligned against the external field, which means if there were protons in that area, they would have a signal at a much lower ppm region. This is opposite to what we mentioned about the outer protons ,which experience the aligned power of external and induced magnetic fields.
Indeed, the outer protons of [18]annulene appear at 8.9 ppm in the 1H NMR spectrum, while the inner protons resonate at -1.8 ppm. This is characteristic of any aromatic system with inner and outer protons:
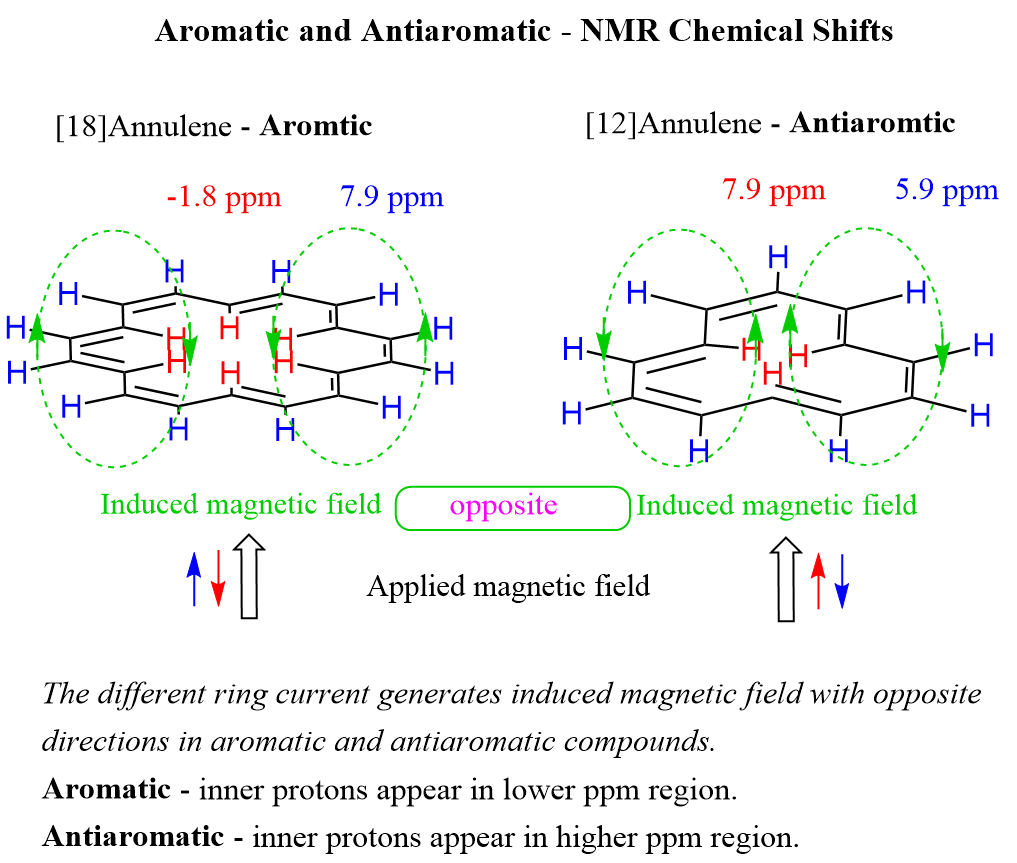
Antiaromatic compounds generate a different ring current, which in turn generates an induced magnetic field with opposite directions from aromatic compounds. Thus, antiaromatic systems show the opposite trend: the inner protons appear in a higher ppm area than the outer protons. For example, the protons outside the ring of [12]annulene appear at 5.91 ppm, whereas the inner protons are characterized by a chemical shift of 7.86 ppm:
Antiaromatic to Aromatic Conversion
Like [12]annulene, [16]annulene also has 4n electrons, and it has been found to be antiaromatic. Interestingly, it can be converted into an aromatic dianion (18 electrons) or a dictation (14 electrons) :
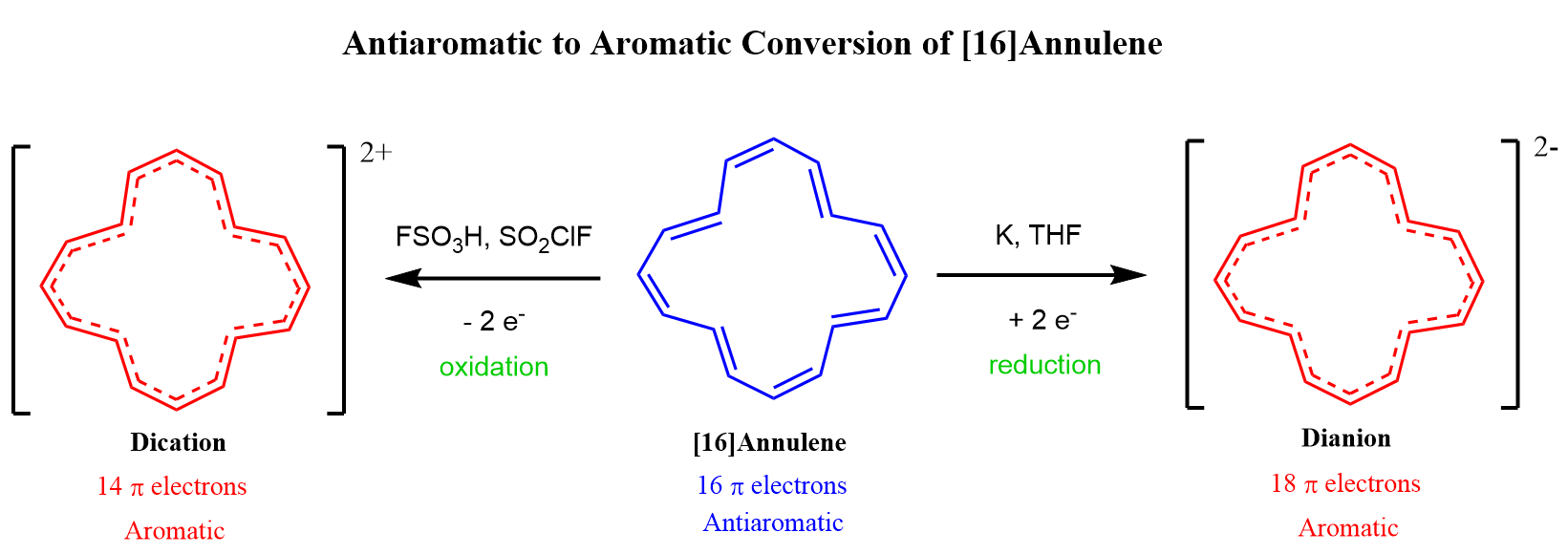
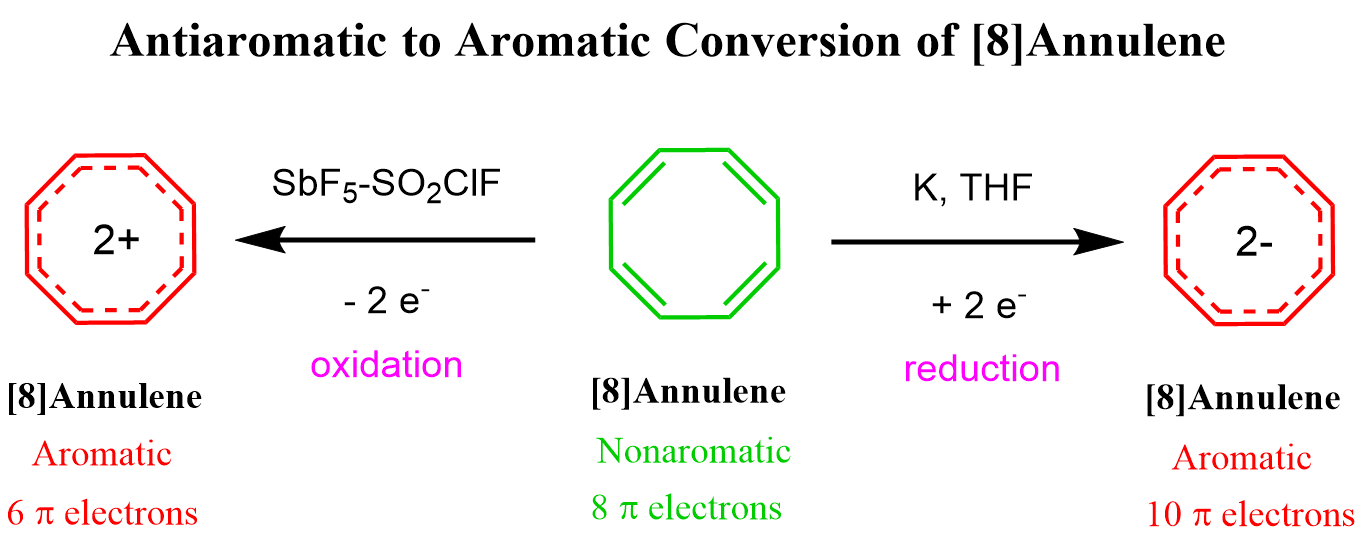
Remember, in the article about Frost circle, we have also seen that cyclooctatetraene can be converted into an aromatic dianion or a dication:
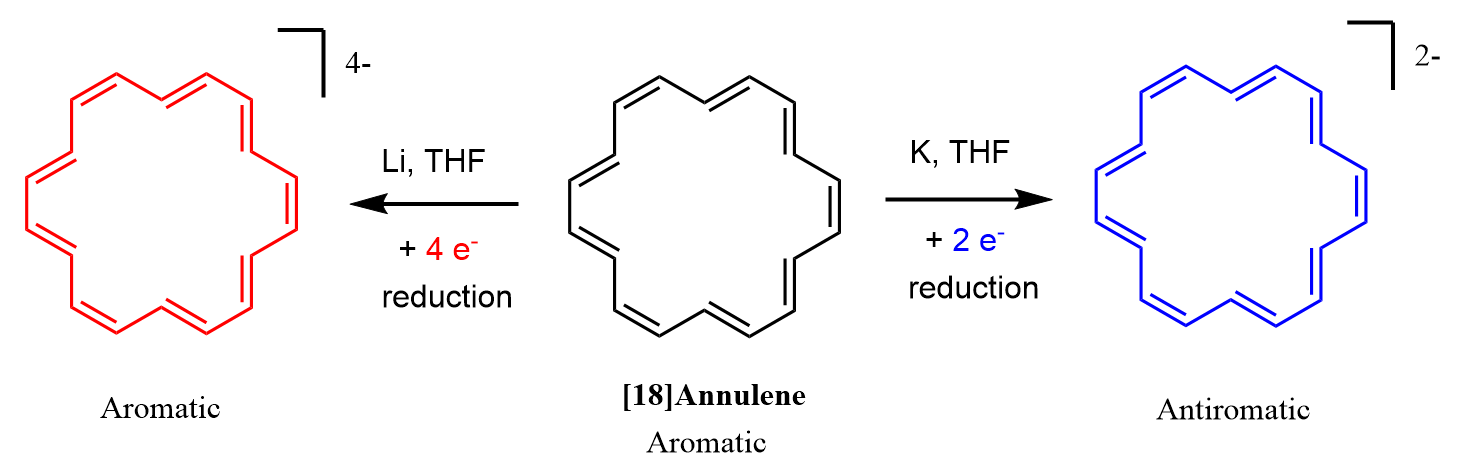
In a recent article in Nature, it has been shown that [18]annulene can be converted into an antiaromatic dianion (20 e–, 4n), and an aromatic tetraanion (22 e–, 4n+2). The dianion is prepared by reduction of [18]annulene with potassium in THF, whereas for the tetraanion, Li in THF is used.
What is fascinating about these compounds is the more profound NMR shift of the inner and outer protons of the rings. The inner protons of the antiaromatic dianion appear at a crazy 32 ppm, while those of the aromatic tetraanion are in the 7-8 ppm region:
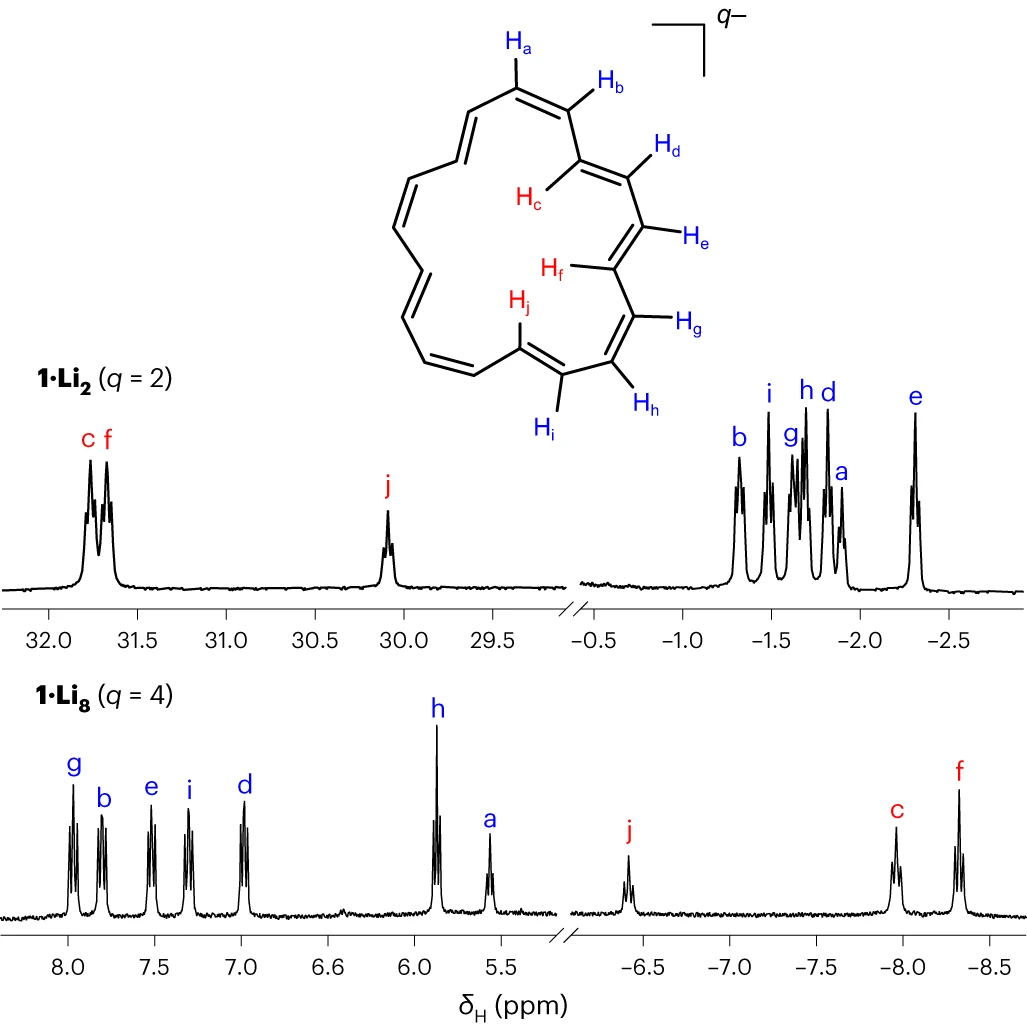
1H NMR spectra of the antiaromatic dianion (top) and aromatic tetraanion (bottom) with Li+ counter ions. THF-d8; temperatures were −70 °C for the dianion and 25 °C for tetraanion; 500 MHz.
Image from Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01469-1
Notice also that the structure of [18] annulene is different from what we showed earlier. This is because they exist in equilibrium, and the number of NMR signals tells us about the symmetry and thus the structure of the compound.
Aside from adding or removing electrons to facilitate antiaromatic-aromatic conversion, other reactions can also be used to, for example, lock the planarity of a ring with 4n+2 electrons. Consider the conversion of cyclodecapentaene to a bridged system 1,6-methano-bridged [10]annulene (J. Am. Chem. SOC. 1995,117, 1369-1373):

I know the structure on the right wouldn’t fit in your example list of planar gentries to explain sp2 hybridization, but you can find more about it in Org. Lett. 2002, 4, 2393.
For further reading, check out the linked articles in the post, and definitely the comprehensive summary on annulenes covered in Chemical Reviews, 2006, Vol. 106, No. 12, 5347 (Renaissance of Annulene Chemistry).
The textbook Organic Chemistry: Structure and Function by Volhard and Schore also has a nice touch on this subject.
Check Also
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Aromaticity and Huckel’s Rule - Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
- Frost Circles
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta in EAS with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators ?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
