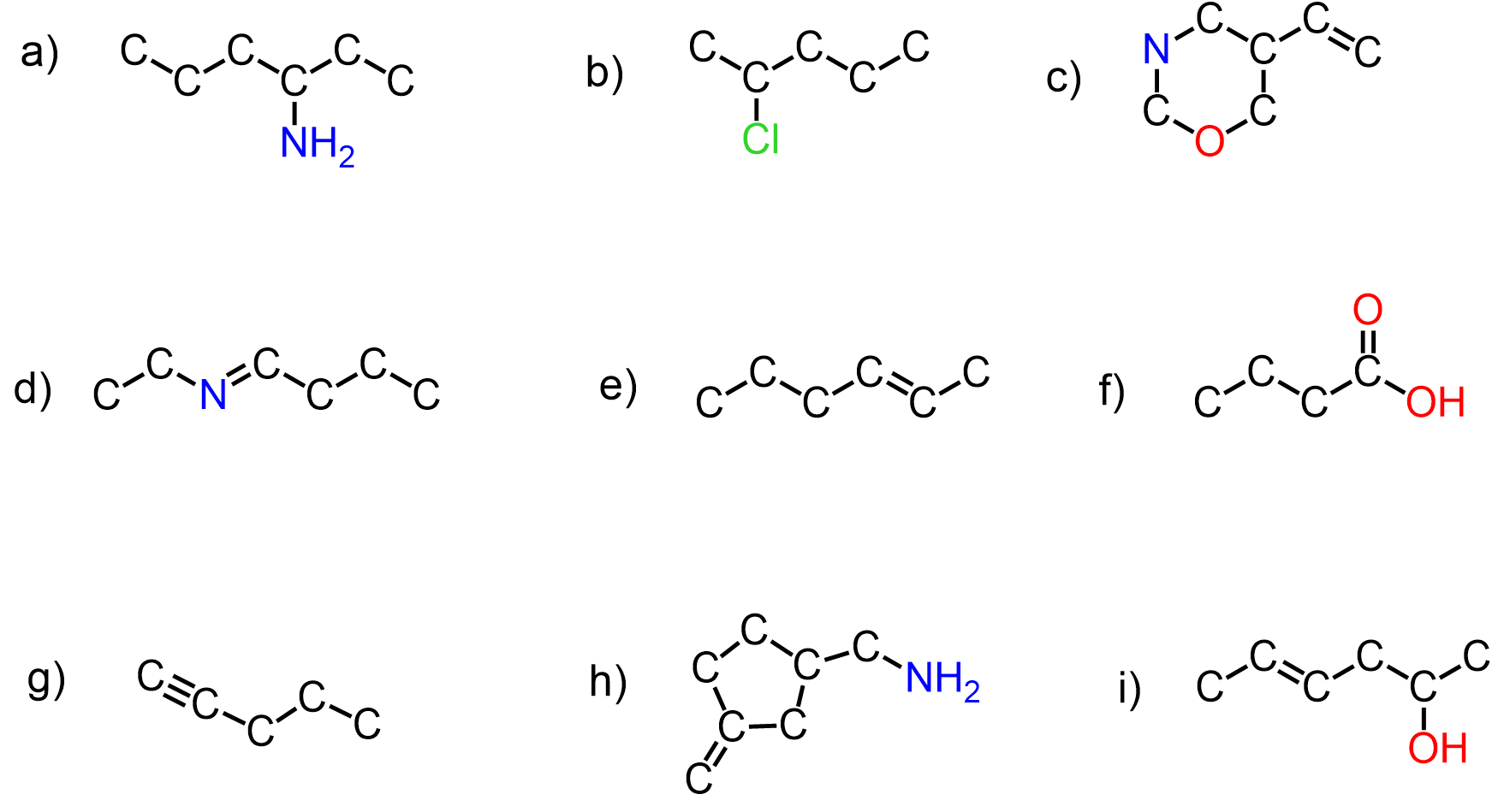
You are in the very beginning of your organic chemistry class, and your professor has told you that carbon has / likes / prefers four bonds. This, of course, is true – the golden number of carbon is 4.
You were also told to never draw carbon with more than four bonds.
Now, you should take this last statement very literally – never show a carbon with five bonds.
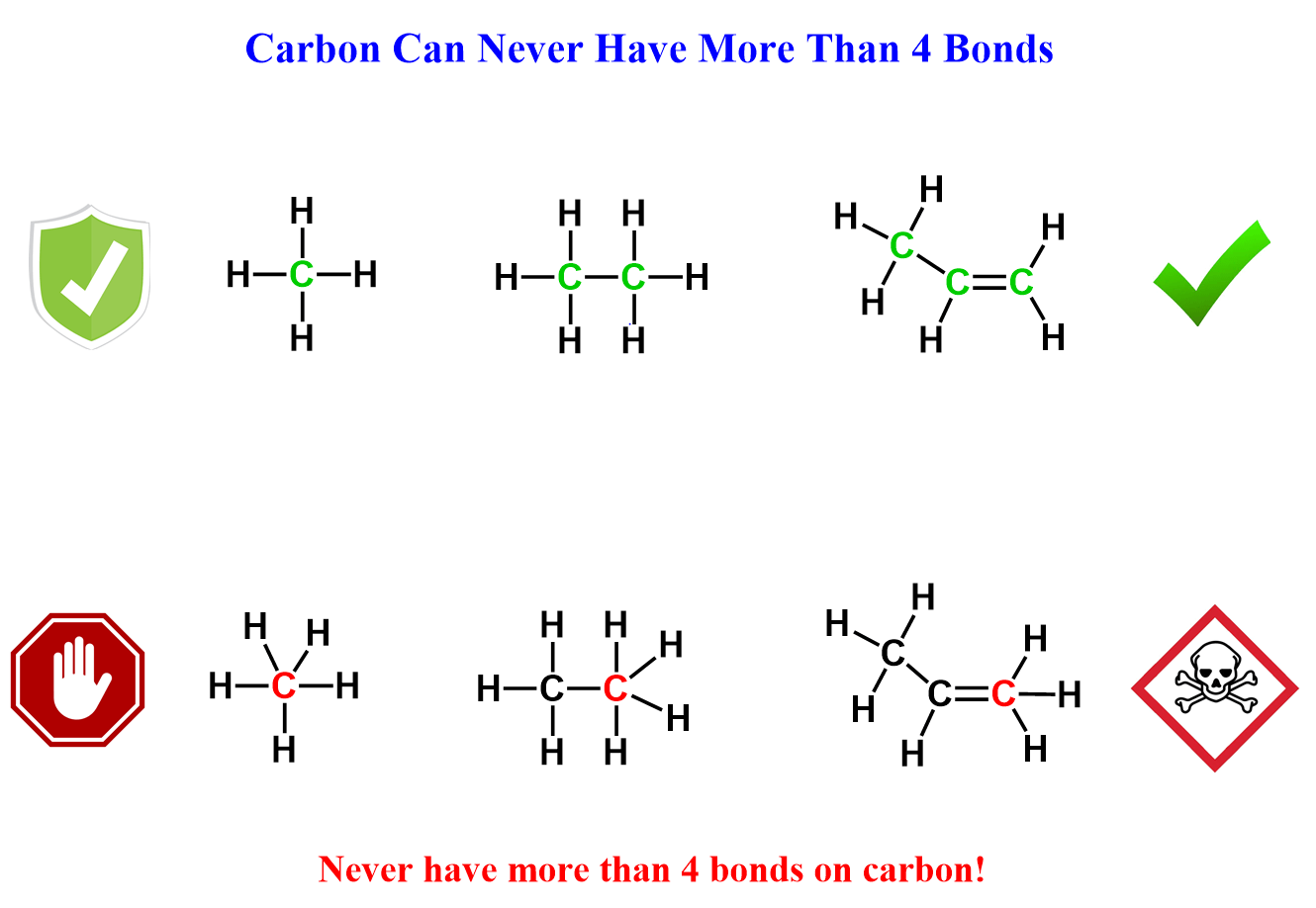
That doesn’t sound too hard, but things get a little trickier when you start using bond-line (zig-zag) notation and drawing resonance structures where the hydrogens connected to carbons are not shown. For example, both of these structures are extremely ugly. Can you find the mistake in each?

In a couple of days or weeks, structures like these should and will hurt your eyes.
If you have not talked about bond-line structures, do not worry about making mistakes in the bonding pattern of carbon. You will get used to them, and with practice, it will become natural to see the number of bonds without having to count them every time.
So, we have answered one question that is never violated in organic chemistry – never draw a carbon with more than four bonds.
Now, the next question requires more explanation:
Can carbon have three bonds?
Let’s get straight to it – yes, carbon can have three bonds, but the real question is, when and how?
Here is what you need to remember: if an atom is off its standard bonding pattern, it must have some lone pair(s) and/or a charge.
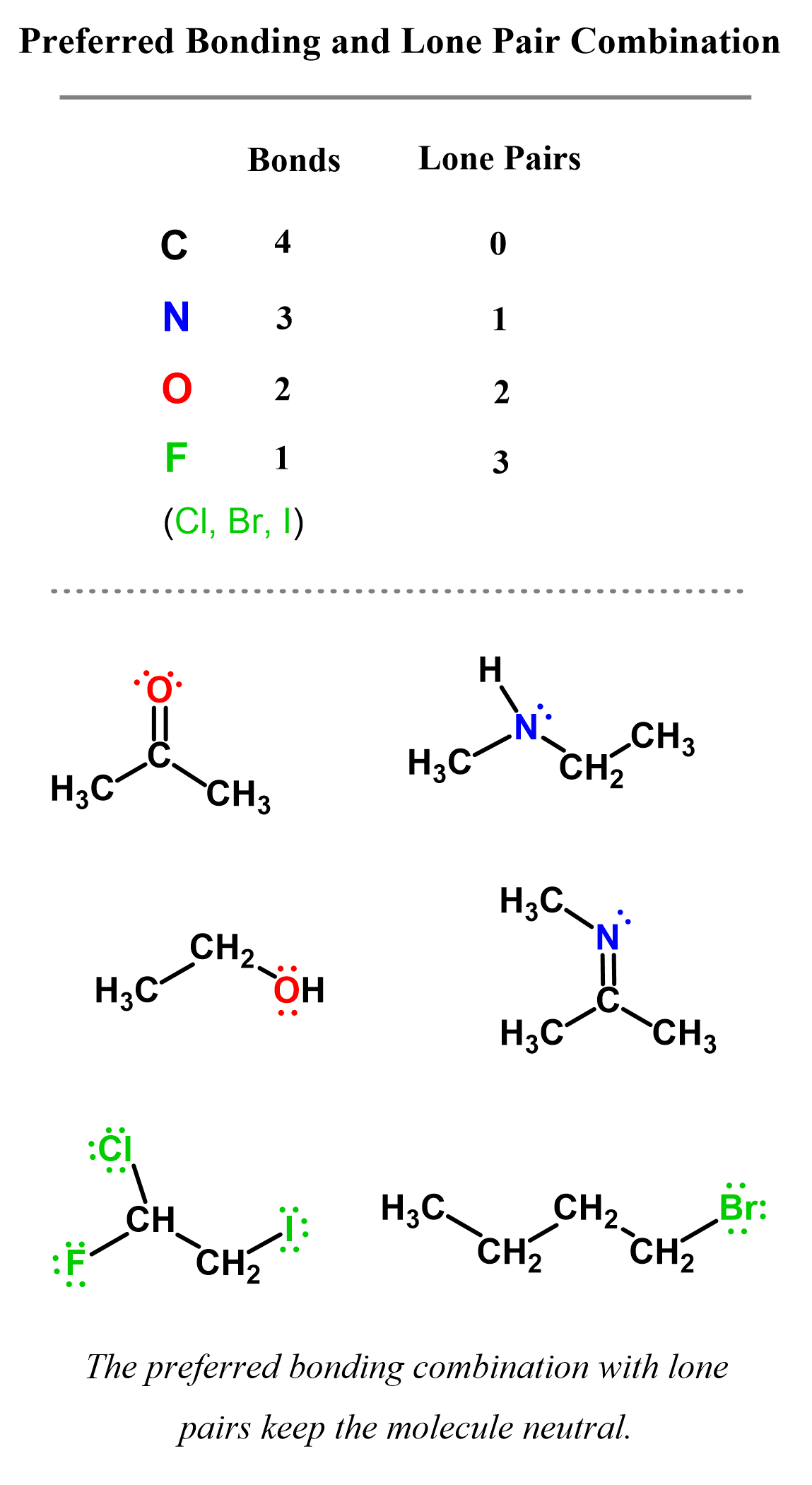
The most important standard number of bonds in organic chemistry are those for carbon, nitrogen, oxygen, and halogens. So, keep these numbers in mind:

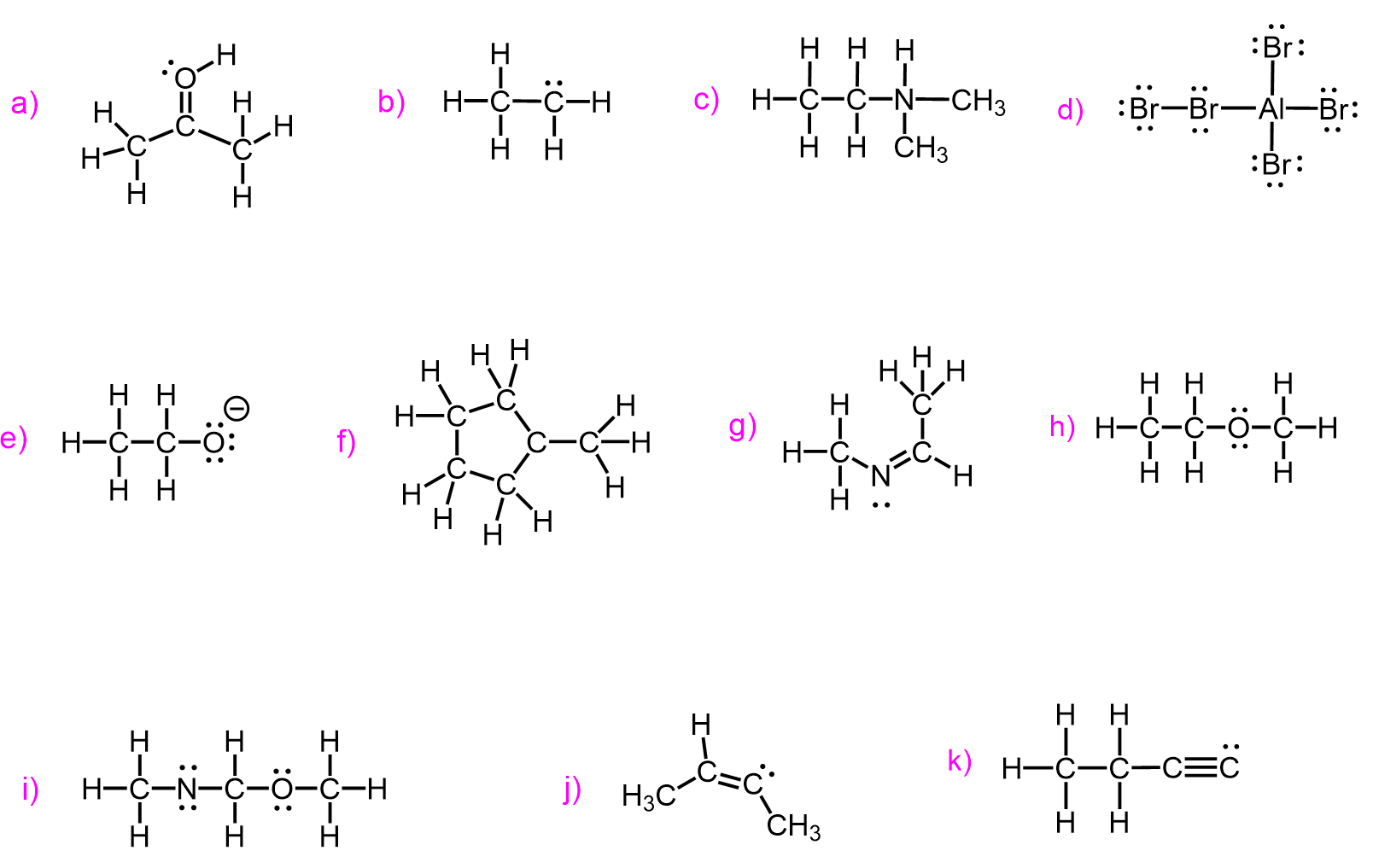
Now, you may also remember from general chemistry that very often atoms have lone pairs too. So, let’s elaborate on this number a little more and add the standard combination of bonding and lone pair for these atoms:

Here are some examples of carbon, nitrogen, oxygen, and halogens in organic molecules where the preferred bonding combination with lone pairs keeps the molecule neutral:
Formal Charges
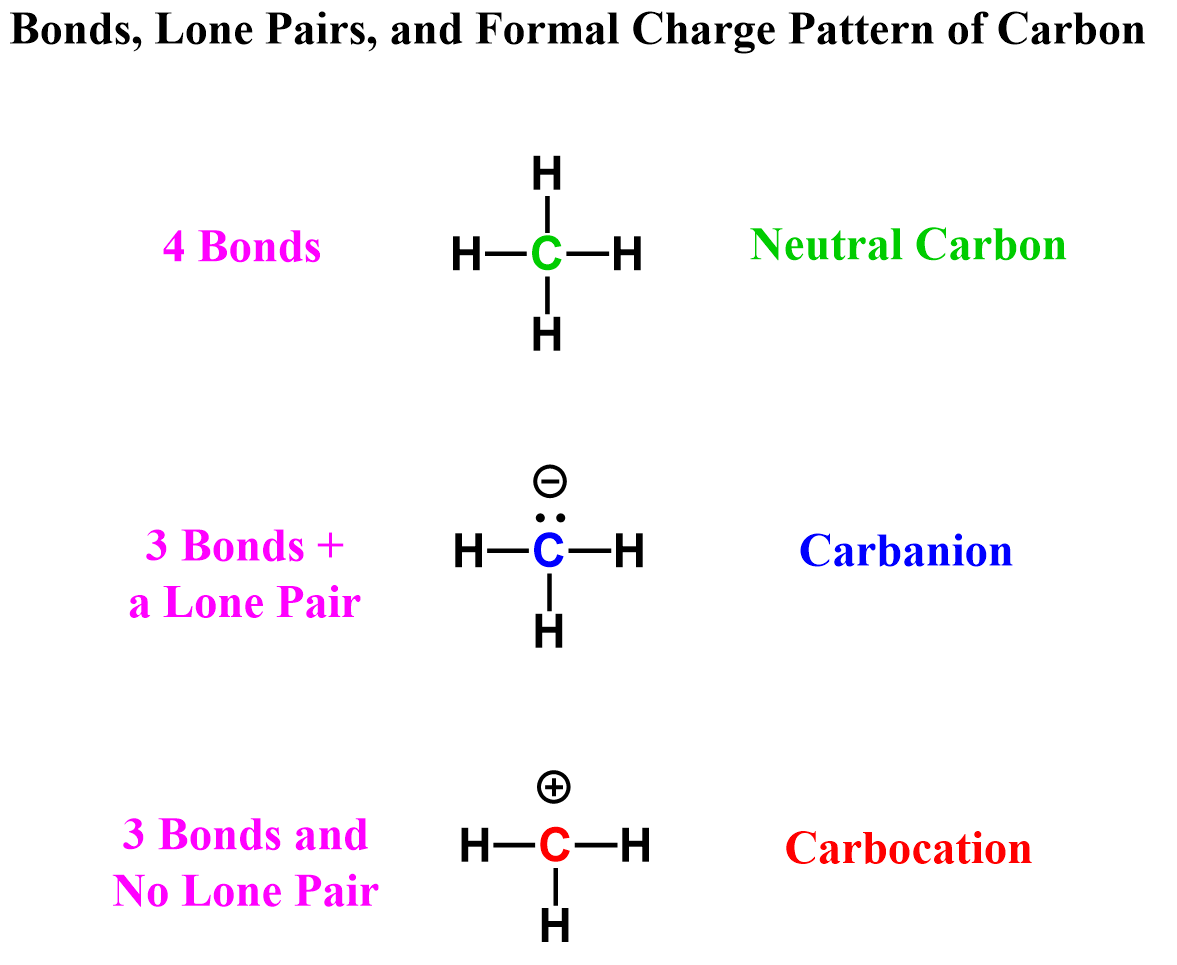
The next factor is formal charges, which become relevant when the atom is off its standard bonding pattern. So, for carbon, that would be when it has three bonds. This can be three bonds with or without a lone pair.
In the first case, the carbon is negatively charged, and we have a carbanion, whereas a carbon with three bonds and no lone pairs is positively charged, thus the name carbocation:
Bonding in Carbocations
What makes carbocations different from carbanions is the lack of octet.
Remember from general chemistry that second-row elements like to have an octet, and they never exceed it. The key word is “they like/prefer” – which does not mean they always do.
What is important here is to know that when carbon (and nitrogen and oxygen) does not have an octet, it is very unstable. Therefore, carbocations are very unstable, which means they are very reactive.
Any species with a pair(s) of electrons will immediately attack this carbon to make a new covalent bond:

This is just an illustration, so do not worry about how the reaction happens, what the arrows are, etc. You will get to this a bit later in the semester.
Notice that the oxygen on the left side is negatively charged because it is off its standard bonding pattern: it has one bond with the hydrogen and three lone pairs which makes its formal charge -1. Once it is bonded to the carbon, it has two bonds and two lone pairs and therefore, it is now neutral.
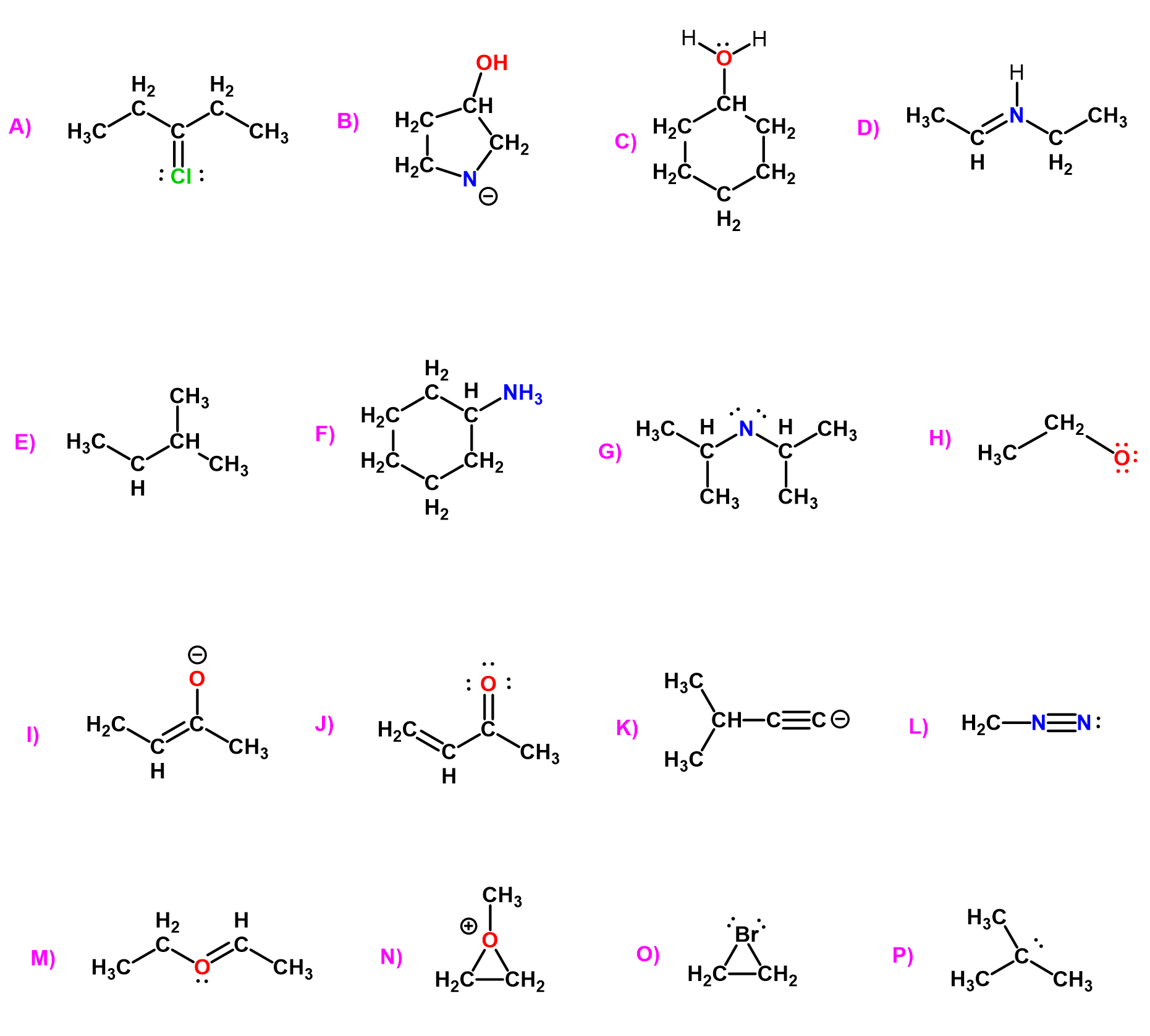
Here are some examples of carbocations and carbanions containing carbon atoms with three bonds. Pay attention to the number of lone pairs and formal charges:
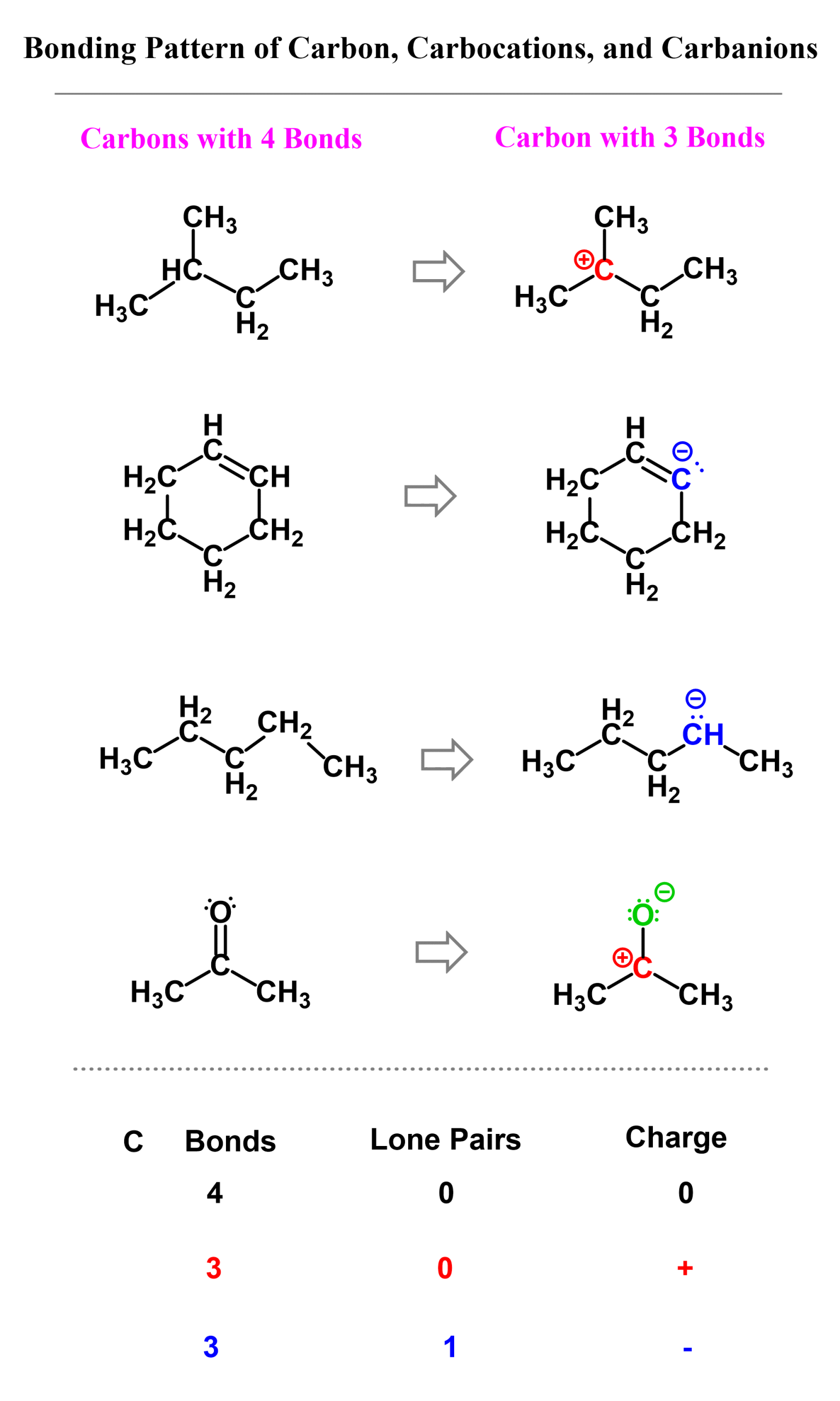
Here is also a table that includes the bonding pattern of nitrogen, oxygen, and halogens in organic chemistry. As a side note, remember that hydrogen can only have one bond.

Bonding in Carbanions
We said that the other possibility of having a carbon with three bonds is when it has a lone pair in addition to those. These are carbanions, and although they follow the octet rule, they are not particularly stable either.
They are electron-rich species, and, opposite to carbocations, are looking for electron-deficient species to react with.
The key information for you here is to remember that neither carbocations nor carbanions are stable. In general, any atom outside of its standard bonding pattern becomes unstable.
Bonding in Double and Triple Bonds?
Although looking at carbon with a double or a triple bond may seem like it has three and two bonds, respectively, this is not true.
Each bond of a multiple bond counts as one, so a double bond is considered two bonds and a triple bond consists of three bonds. In other words, each pair of shared electrons counts as one bond, so a double bond has two shared pairs (two bonds), and a triple bond has three. Remember, in each multiple bond, we have one sigma (σ) and the rest are pi (π) bonds.
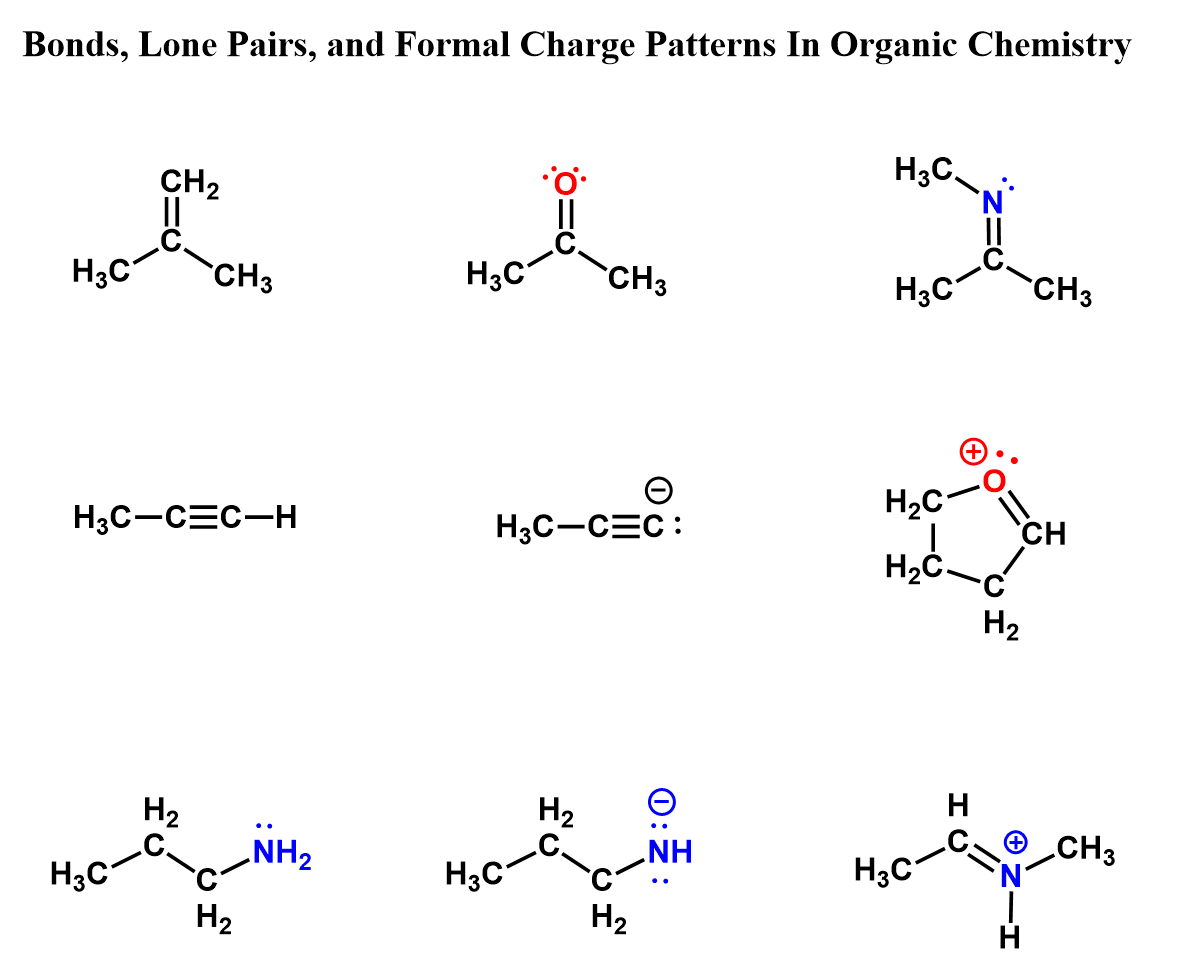
Here are some examples of carbon, nitrogen, and oxygen having different combinations of bonds, lone pairs, and formal charges:
Summary: Bonding Patterns in Organic Chemistry
Understanding bonding patterns is one of the most important foundations in organic chemistry. We have discussed the standard bonding preferences for carbon, nitrogen, oxygen, and halogens and how lone pairs and formal charges come to the rescue when these are not followed.
Key points to remember:
- Carbon prefers four bonds, and you should never draw it with five.
- When an atom doesn’t follow its standard bonding, it usually comes with a formal charge and/or a lone pair.
- Carbocations (electron-deficient) lack an octet, and carbanions (electron-rich) are both unstable and highly reactive.
- Bond-line structures may hide hydrogens, but the bonding pattern is always followed.
- Double and triple bonds count as two and three bonds, even though they may look simpler in drawings.
In the end, keep in mind that you’ll only master this by doing lots of practice, and in organic chemistry, practice means drawing structures. So, a pencil and paper should be your best friends. Don’t worry about getting stuck or making mistakes – nobody is judging you. It’s better to make them here than on the test.