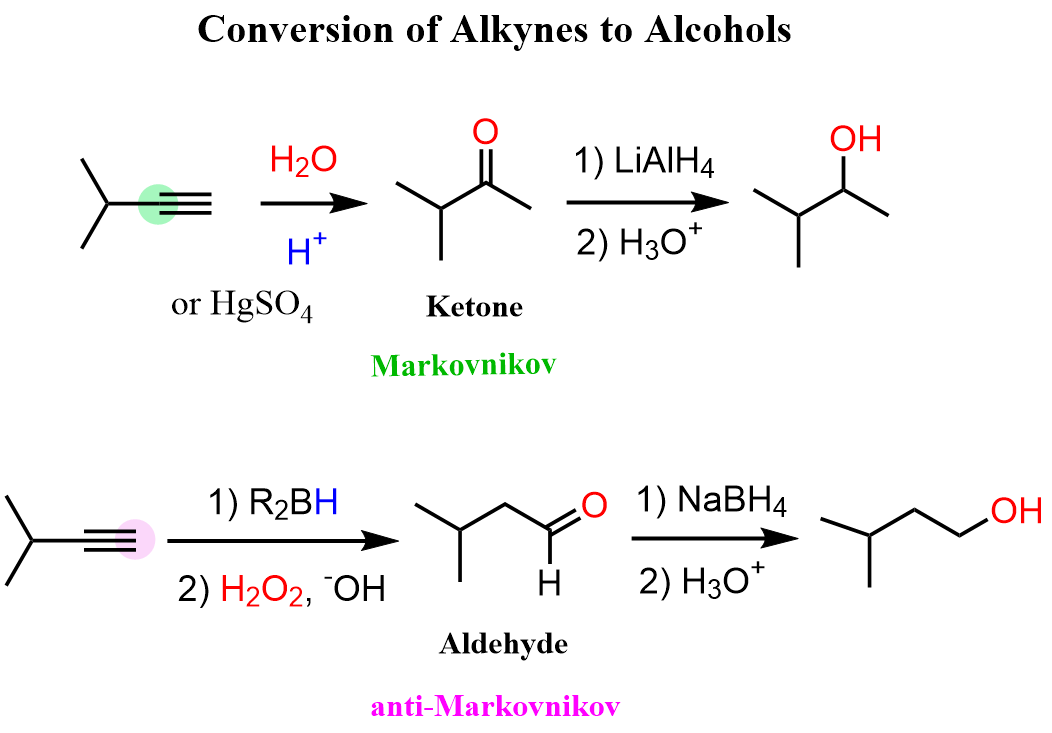
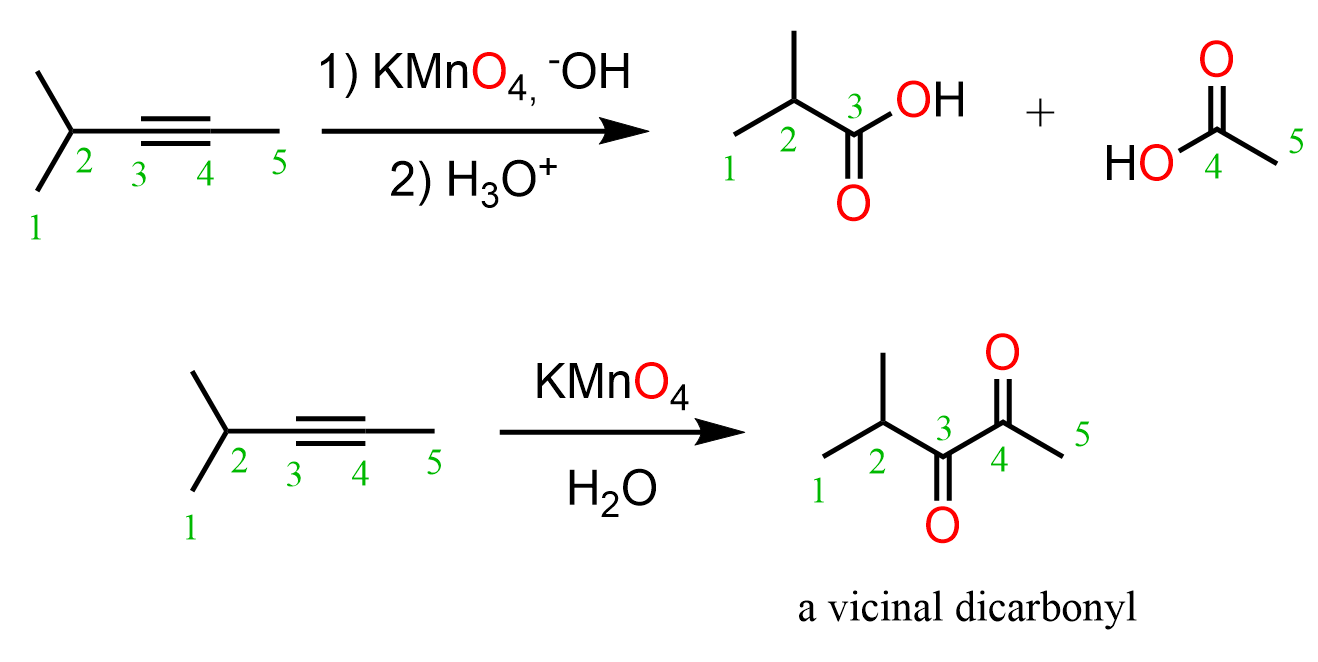
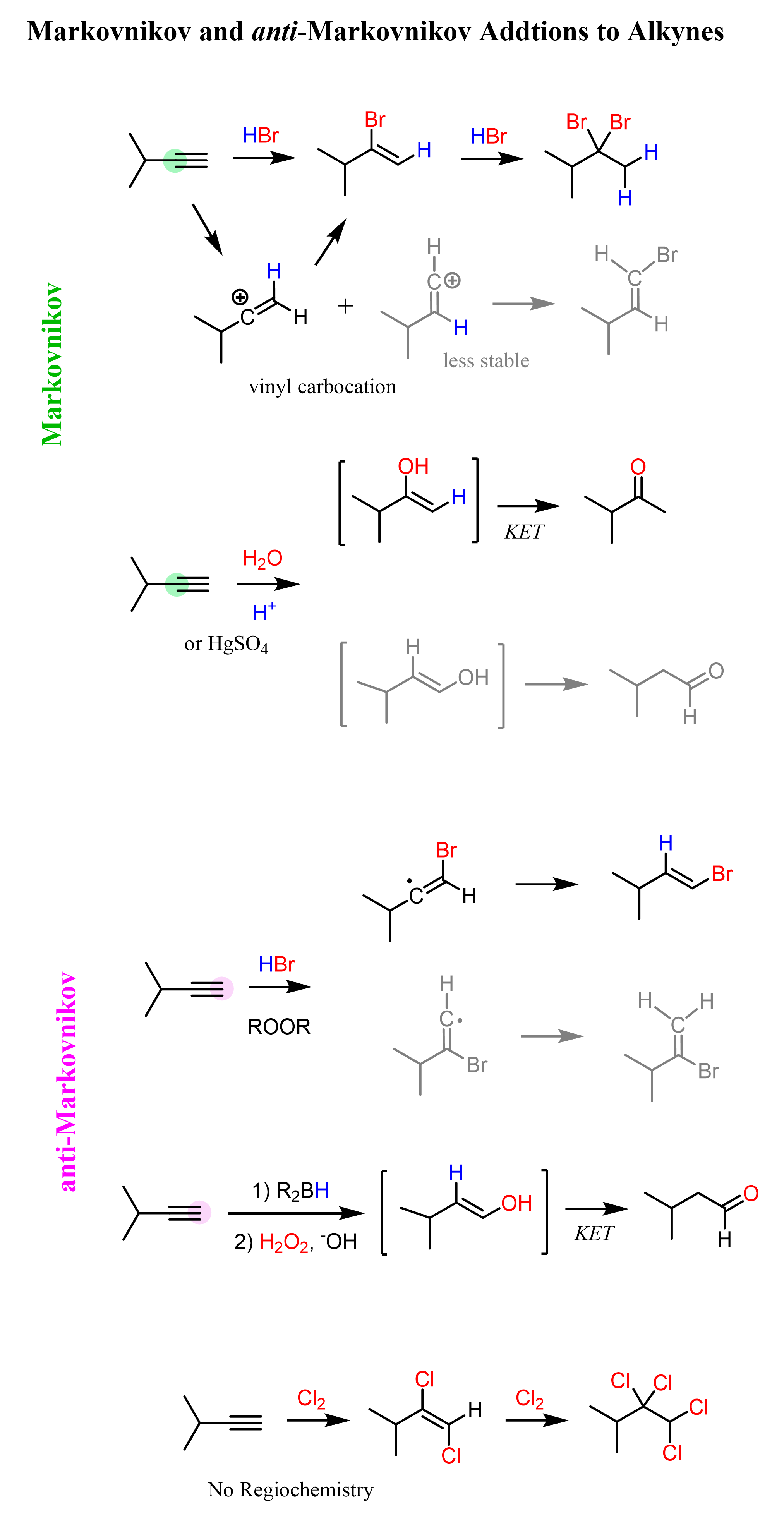
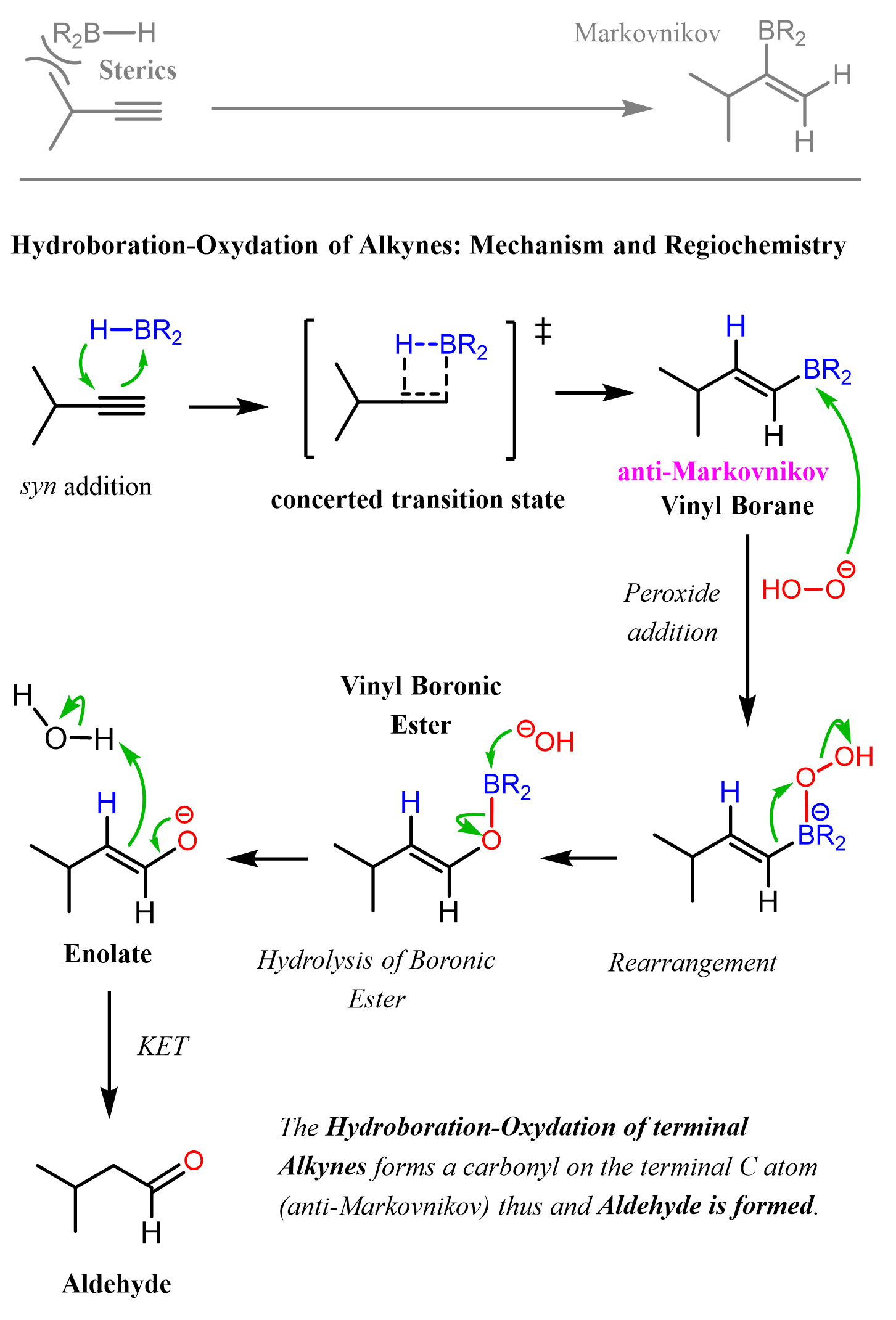
Alkynes to Alcohols
When alkynes are reacted with water, aldehydes and ketones are formed. While internal alkynes can only be converted to ketones by acid-catalyzed hydration or oxymercuration-demercuration, terminal alkynes can also be converted to aldehydes via anti-Markovnikov addition of hydroboration-oxidation: … Read more