Cis and Trans Alkenes
Alkenes can be stereoisomeric even though this is slightly different than what we learn about enantiomers and diastereomers containing chirality centers. For example, let’s try to determine the relationship between molecules in each of the following pairs:
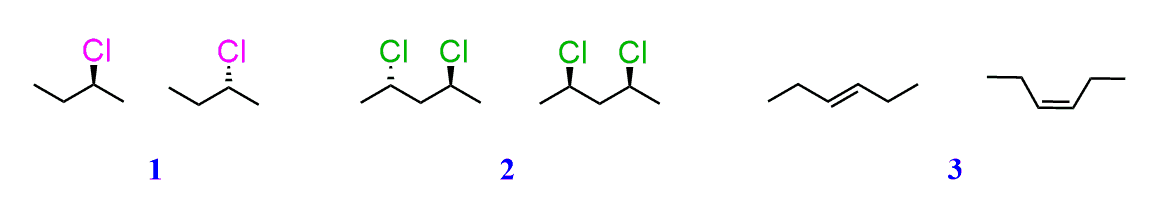
The first two molecules are a pair of enantiomers since they are nonsuperimposable mirror images. The second pair represents diastereomers since they are stereoisomers but not mirror images:

So, what is the relationship between the two alkenes?
They are clearly not identical because the atoms on the double bond are pointing at different directions. They are also not constitutional isomers since all the atoms are connected the same way.
They are not mirror images either and the only option is to classify them as diastereomers since they are stereoisomers:

To distinguish this specific type of diastereomers, the cis and trans designation is used.
And to determine if the alkene is cis or trans, you need identify the two identical groups that are connected to each carbon of the double bond. In this case, this are the two ethyl groups:
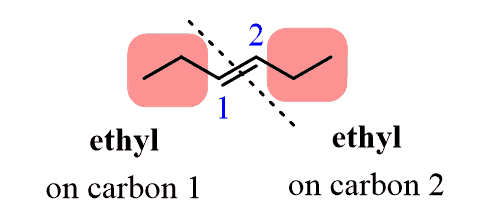
If these groups are on the same side of the double bond, then it is cis and if they are on opposite sides of the double bond, then we have a trans isomer:

The cis and trans designation is included in the nomenclature of alkenes to distinguish the stereochemistry.
The cis and trans designation is not determined based on alkyl groups only.
For example, 2-pentene is not a symmetrical molecule thus we cannot have two identical alkyl groups on both carbons of the c=c bond:
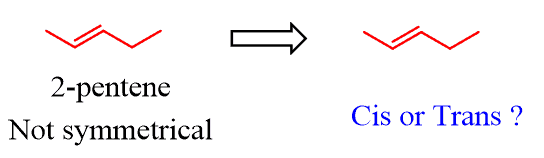
However, remember that they are hydrogens that are not shown since it is a bond-line structure.
If we draw out these hydrogens, it becomes evident that they can be cis or trans just the alkyl groups in 3-hexene:
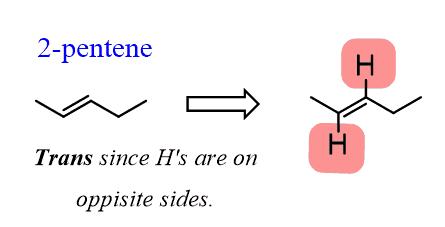
To summarize, as long as there are two identical groups (atoms) on both carbons of the double bond, the alkene can be classified as cis or trans.
Non-Stereoisomeric Alkenes
One important thing to emphasize about the identical groups: these groups must be on each carbon atom of the double bond! If any of the carbons in the double bond is connected to two identical groups, the alkene cannot be cis or trans. That is to say, the alkene cannot be stereoisomeric.
For example, the following alkene has two ethyl groups on the right carbon, and we cannot say that there are two ethyl groups on the same or on the opposite sides of the double bond:
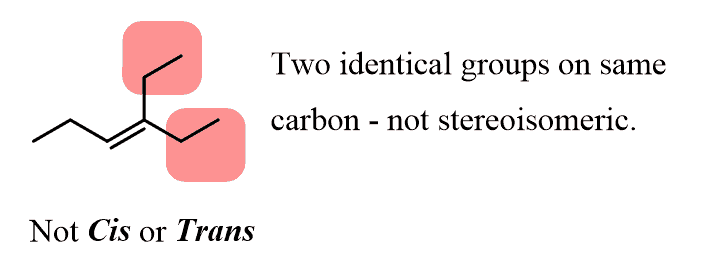
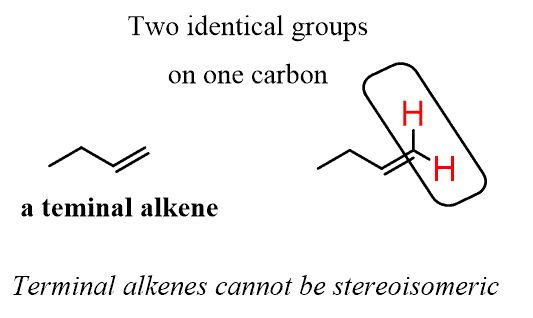
The same limitation applies to terminal alkenes (the double bond in on the first two carbons).
For example, in the following alkene, the carbon is connected to two hydrogens, thus the alkene cannot be cis or trans:
The cis and trans designation is not a comprehensive solution for internal alkenes either. It only works when two identical groups are present on the two carbons of the double bond.
To illustrate this, let’s consider two isomeric alkenes having four different groups on the double bond:

Since there are no identical groups, we cannot classify this as cis or trans and here the E and Z designation is used which we will cover in the next post.
Cis and Trans Isomerism of Cycloalkanes
The origin of the cis and trans isomerism is the “locked” feature of the double. It locked because there is no rotation around the double bond and this, in turn, means that we cannot switch the orientation of the groups on the double bond.
Recall that there is a free rotation about sigma bonds and that is the origin of conformers:

Confirmations represent the same compound and this conversion is possible because of rotation about sigma bonds. A more detailed coverage of this topic can be found in this article.
On the other hand, the different arrangement of atoms on the double bond cannot be changed since the atoms are locked-there is no rotation about a double bond. And that is the configuration of the double band that cannot be changed just like the R and S configuration of a chiral carbon atom. Therefore, cis and trans isomers are stereoisomers.
This comparison brings an interesting question of whether we can ever have cis–trans isomerism for a system with single bonds.
And the answer is yes, there are cis and trans isomers for systems with sigma bonds.
The restricted rotation about the single bonds in cyclic systems makes it possible to distinguish cis and trans isomers depending on the relative orientation of the groups connected to the ring.
The substituents of the cis isomer are on the same side of the ring while the trans isomer has them on opposite sides of the ring:

A solid wedge line represents indicates a bond pointing toward the viewer and a dashed line represents a bond pointing away from the viewer.
Sometimes, the cis and trans are used even if the two groups on the ring are not identical:

And this is similar to what we discussed about double bonds with different alkyl groups. Remember, in this case we focus on the orientation of hydrogens. If both hydrogens are on the same side, it is cis and if they are on opposite sides, then we have a trans isomer.

Nucleophilic Substitution and Elimination Practice Quiz
Check Also
- Is it SN1 SN2 E1 or E2 Mechanism With the Largest Collection of Practice Problems
- General Features of Elimination
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- Elimination Reactions of Cyclohexanes with Practice Problems
- The E1 Mechanism: Kinetcis, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- Stereoselectivity of E1 Reactions
- Nucleophilic Substitution vs Elimination Reactions
- Regioselectivity of E1 Reactions
- E2 vs. E1 Elimination Mechanism with Practice Problems
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Is it SN1 SN2 E1 or E2 Mechanism With the Largest Collection of Practice Problems
- General Features of Elimination
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- Elimination Reactions of Cyclohexanes with Practice Problems
- The E1 Mechanism: Kinetcis, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- Stereoselectivity of E1 Reactions
- Nucleophilic Substitution vs Elimination Reactions
- Regioselectivity of E1 Reactions
- E2 vs. E1 Elimination Mechanism with Practice Problems



Thank you for this valuable lesson