In the previous few posts, we talked about the stability of aromatic compounds and the rules for determining whether a compound is aromatic or not. Aside from experimental data, the stability of aromatic compounds is also confirmed by sophisticated math calculations which we, organic chemists, do not like so much, and any tools that cut this path are much appreciated. One of these tools is the Inscribed Polygon Method also known as the Frost Circle. It relies on the stability of molecular orbitals (MO) where the main feature you need to remember is that the bonding molecular orbitals are the lowest in energy (most stable), followed by nonbonding and antibonding orbitals:
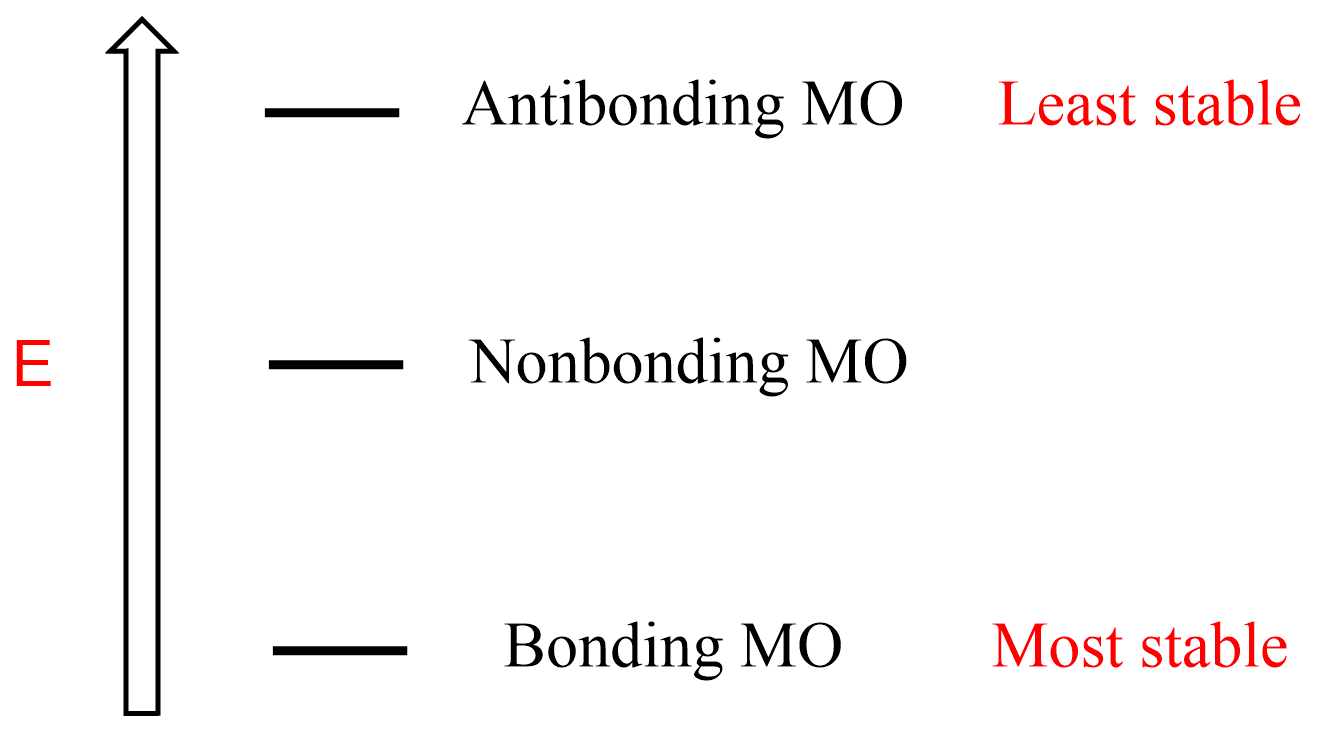
In general, the more electrons we have in the bonding orbital, the more stable the molecule is. Importantly, the opposite is true for the nonbonding, and especially antibonding orbitals – the less they are occupied by electrons, the greater the stability of the molecule:
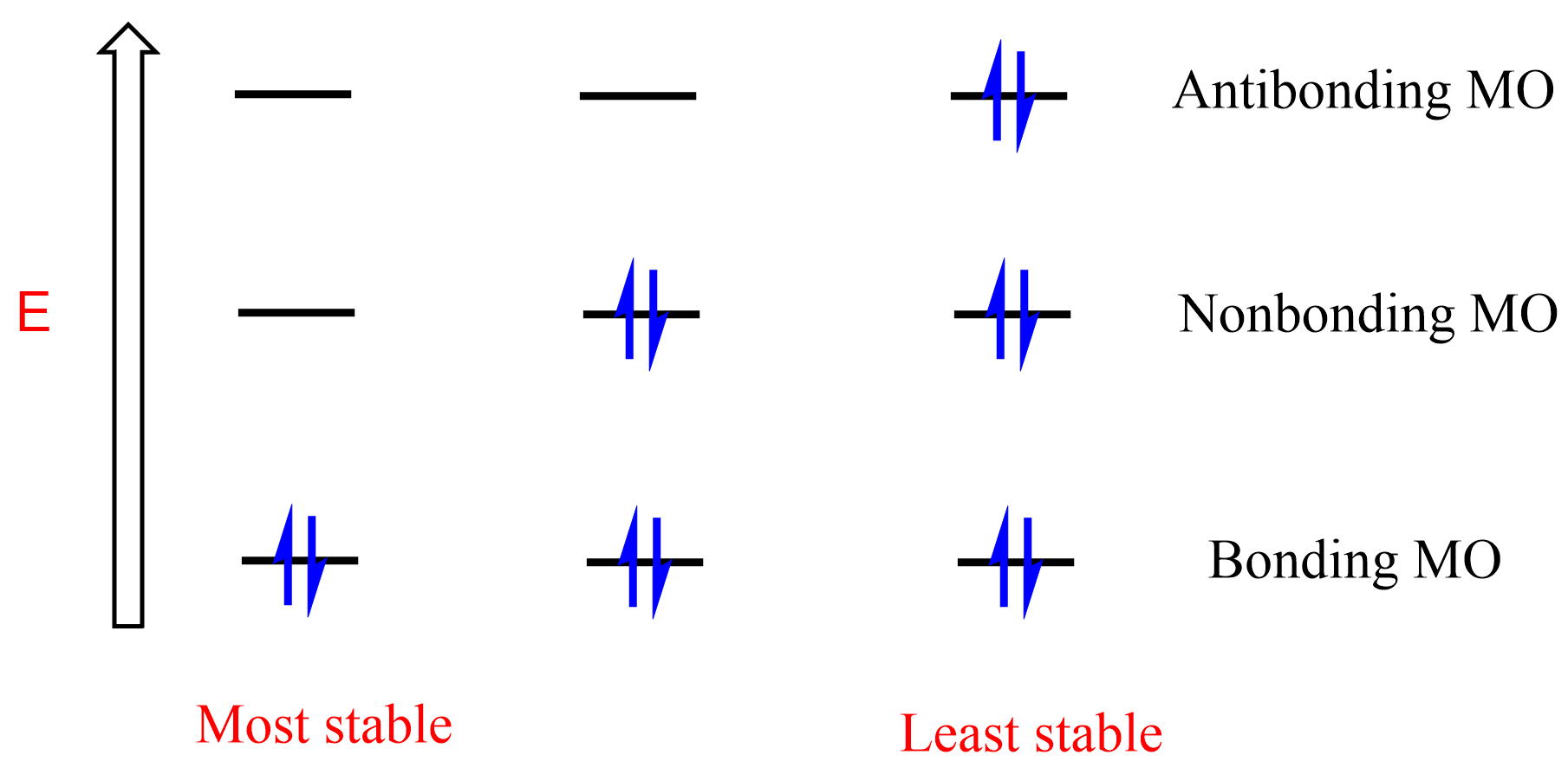
One of the challenges with the MO theory is it gets complicated identifying the bonding, nonbonding, and antibonding orbitals as the molecules get larger. For example, how does one quickly draw the MO diagram of benzene and cyclooctatetraene and to show that the first is aromatic while the second is not? This is where the Frost circle becomes handy.
How to Draw Frost Circles
Let’s start this with benzene – the most common, six-membered, aromatic hydrocarbon.
1) Draw the polygon (benzene – hexagon, cyclopentadiene – pentagon and etc.) pointing one of the vertices down:
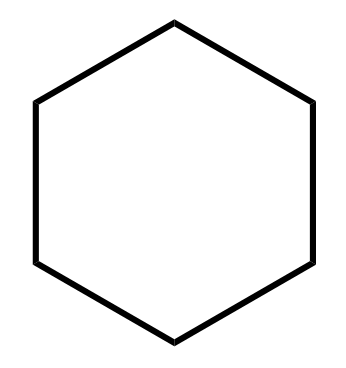
2) Add a circle around the polygon such that all the vertices touch it.
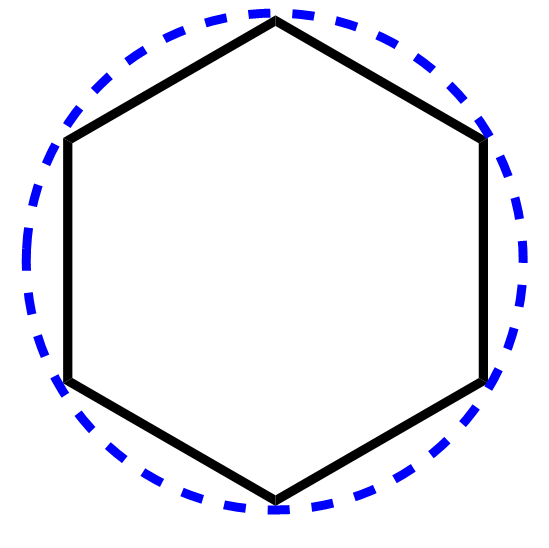
We are inscribing the polygon into the circle by pointing one of the vertices down and making sure they all intersect the circle. The order of steps 1 and 2 is not important, so you can do as you find it easier.
3) Draw a horizontal line passing right in center of the circle.
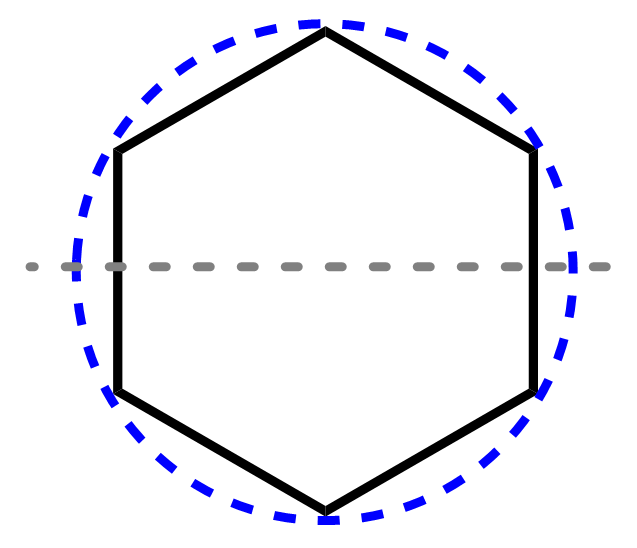
4) Mark the points at which the polygon intersects the circle. The ones below the central line are the bonding orbitals. The line represents the energy level of nonbonding orbitals (if there are any), and the ones above it are the antibonding orbitals:
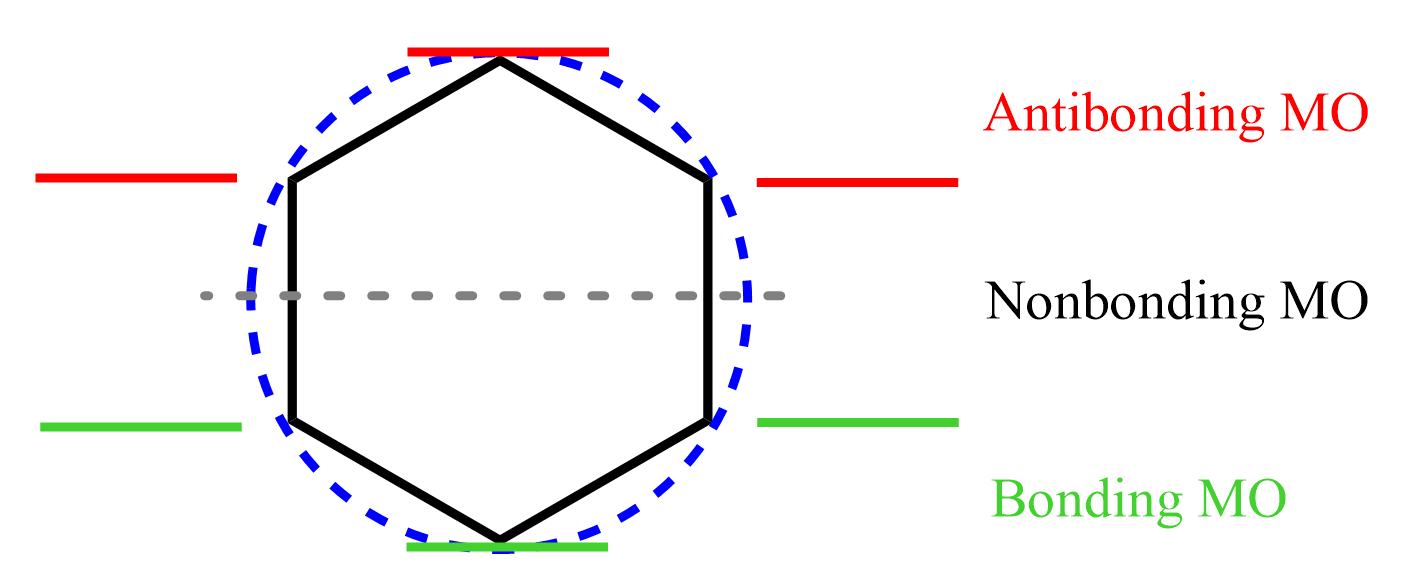
Notice that the total number of MOs equals the number of vertices of the polygon, and this is true for any molecule applicable to study with the Frost circle method.
5) Fill in the orbitals with electrons starting from the bottom – the lowest energy bonding orbital. Benzene has 6 π electrons, so they all go to the bonding orbitals:

Aromatic, Antiaromatic, and Frost Circle
We have drawn the Frost circle for benzene, and now, the most important question: when is the molecule aromatic according to the Frost circle?
Aromaticity is achieved when all the bonding orbitals are filled with paired π electrons, while no unpaired electrons are in the nonbonding and antibonding orbitals. Having unpaired electrons, even in the bonding orbitals, often indicates an antiaromatic compound because of the radical nature of the electron configuration. Can we make this even shorter? Perhaps, yes, because the electrons always fill the bonding orbitals first, and there is always enough of them to do that, so the only thing that really matters is whether there are unpaired electrons or not.
The requirement of having completely filled bonding MOs, and no unpaired electrons confirms the aromaticity of benzene. We can use the Frost circle for all monocyclic, completely conjugated systems regardless of the ring size. For example, let’s see why cyclobutadiene is not aromatic:
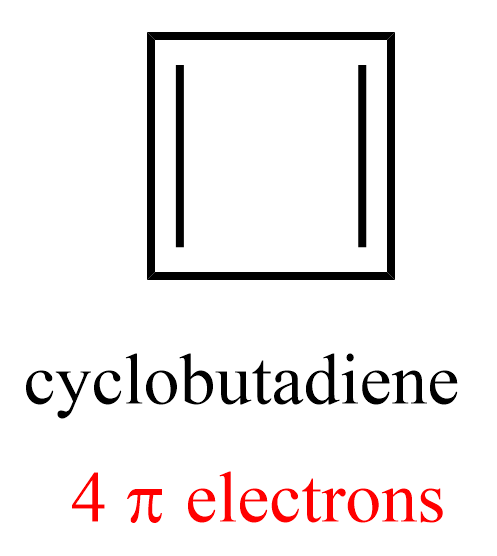
Following the steps listed above, we inscribe a square in a circle, with a vertex pointing down:
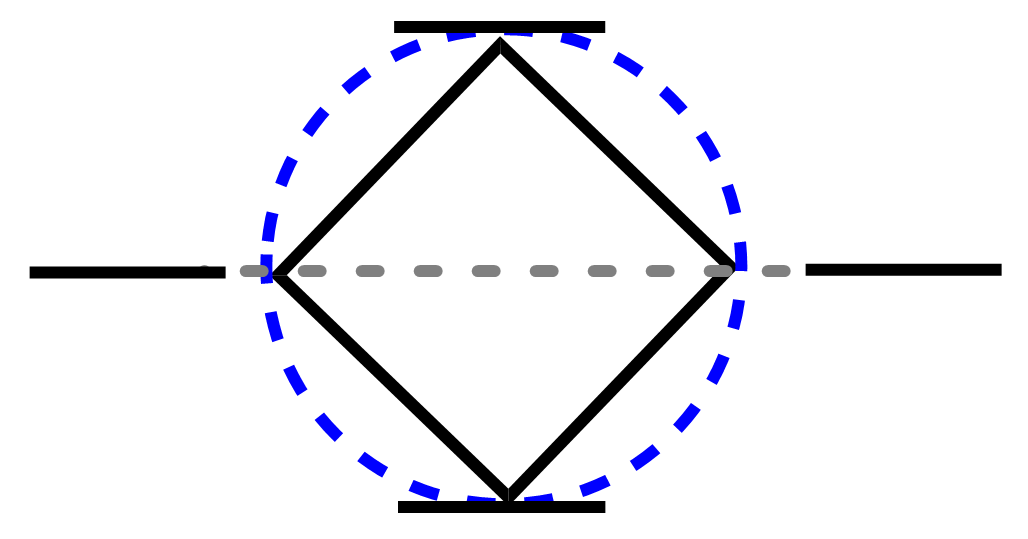
The four intersecting points represent one bonding, two nonbonding, and one antibonding molecular orbital. Cyclobutadiene has four π electrons, two of which go in the bonding MO, and two to the nonbonding orbitals:
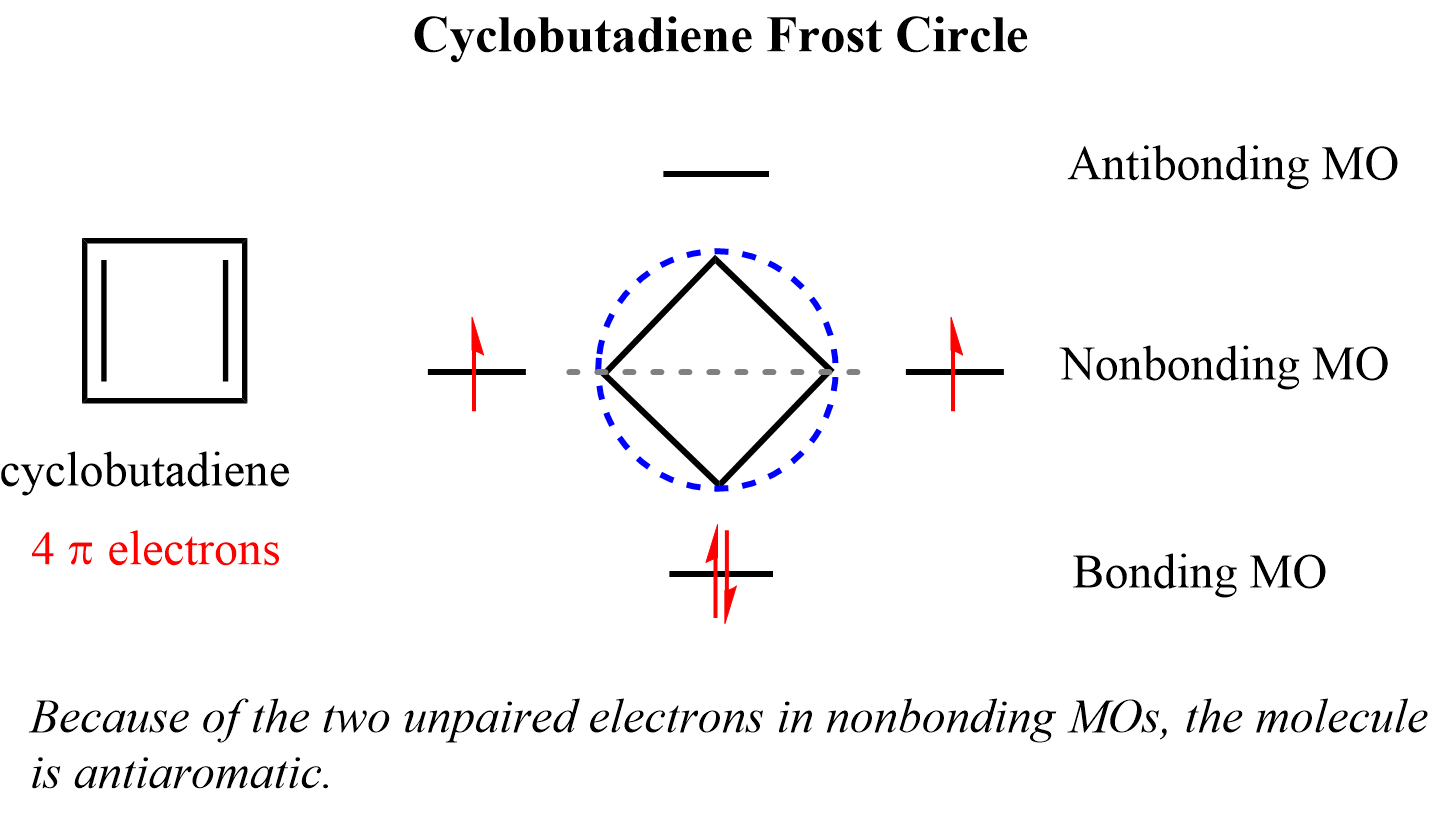
Notice that the two electrons in the nonbonding orbitals are unpaired (Hund’s rule) which makes it highly reactive/unstable diradical. Unpaired electrons in high-energy orbitals are a common trait in antiaromatic compounds.
Is Frost Circle an Ultimate Indicator for Aromaticity?
Like most theories, the Frost circle method is used to explain experimental observation and is not a sole and ultimate tool to classify a molecule as aromatic or antiaromatic.
A good example such example is cyclooctatetraene – a fully conjugated cyclic molecule with 4n electrons predicted to be antiaromatic. Two out of its eight electrons go unpaired in the nonbonding orbitals, and one might predict the molecule to be antiaromatic:
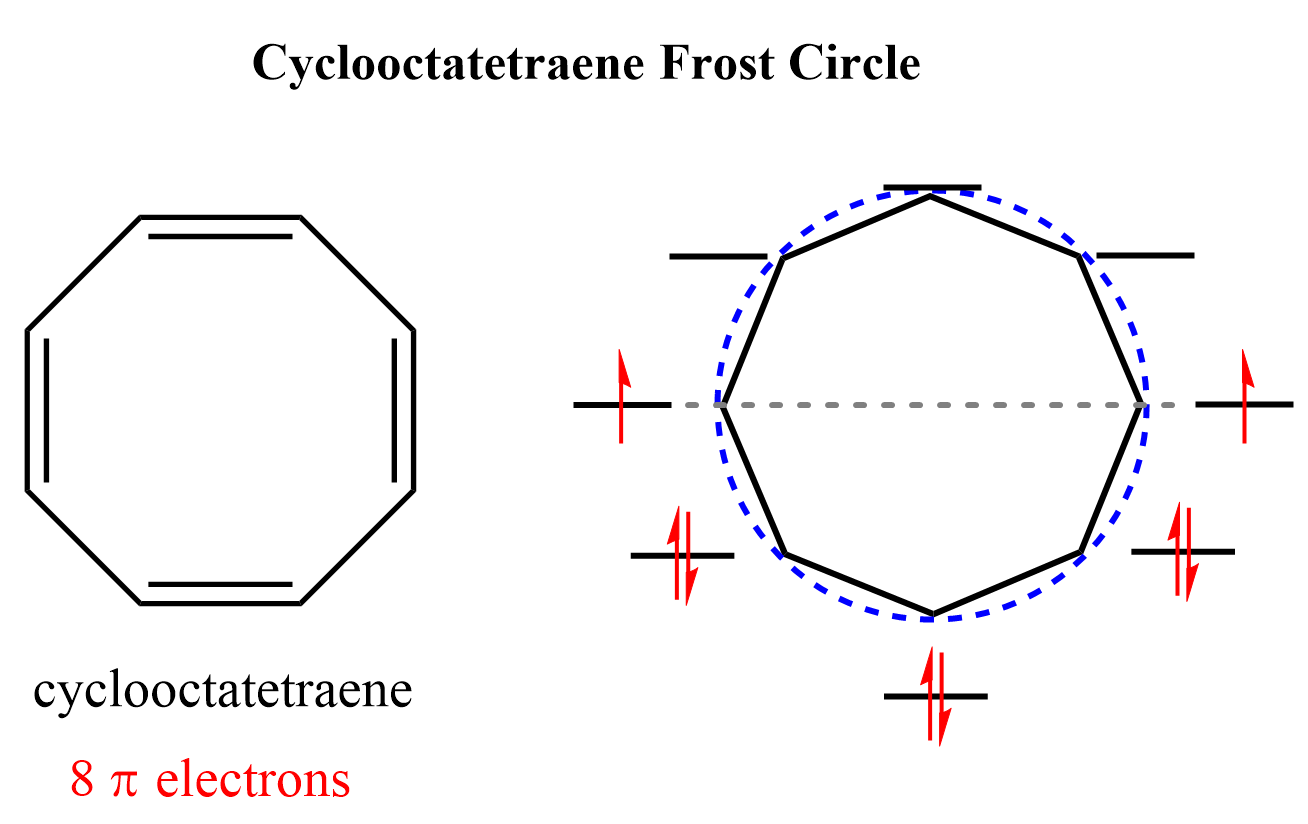
However, recall from the previous post that it avoids being antiaromatic by adopting a nonplanar geometry, and falls into the category of nonaromatic compounds:

This also is a common trend – if the molecule has a way of avoiding being antiaromatic, it will do it.
We can either add (10 e–) or remove two electrons (6 e–) from cyclooctatetraene making diionic aromatic species which meet the requirements of Huckel’s rule and Frost circle.
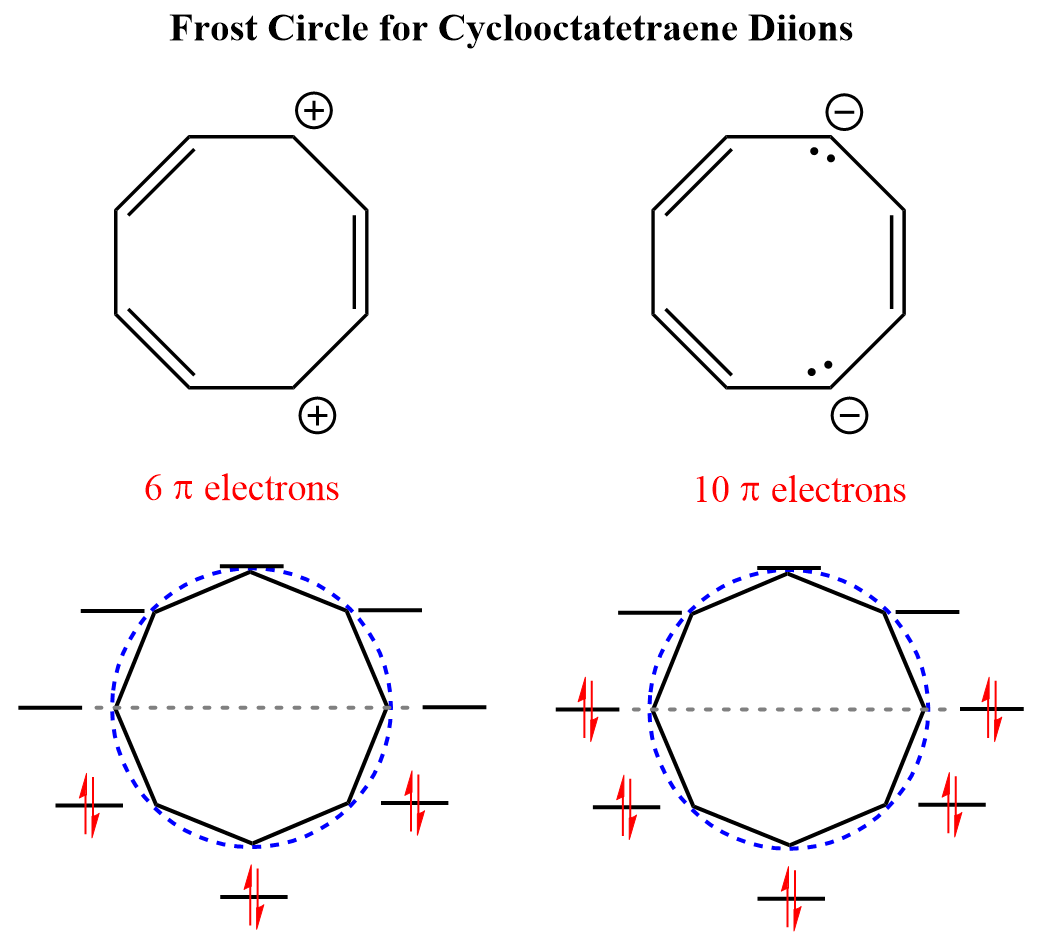
Interestingly, both the dication and dianion compounds have been synthesized and confirmed to have aromatic character:
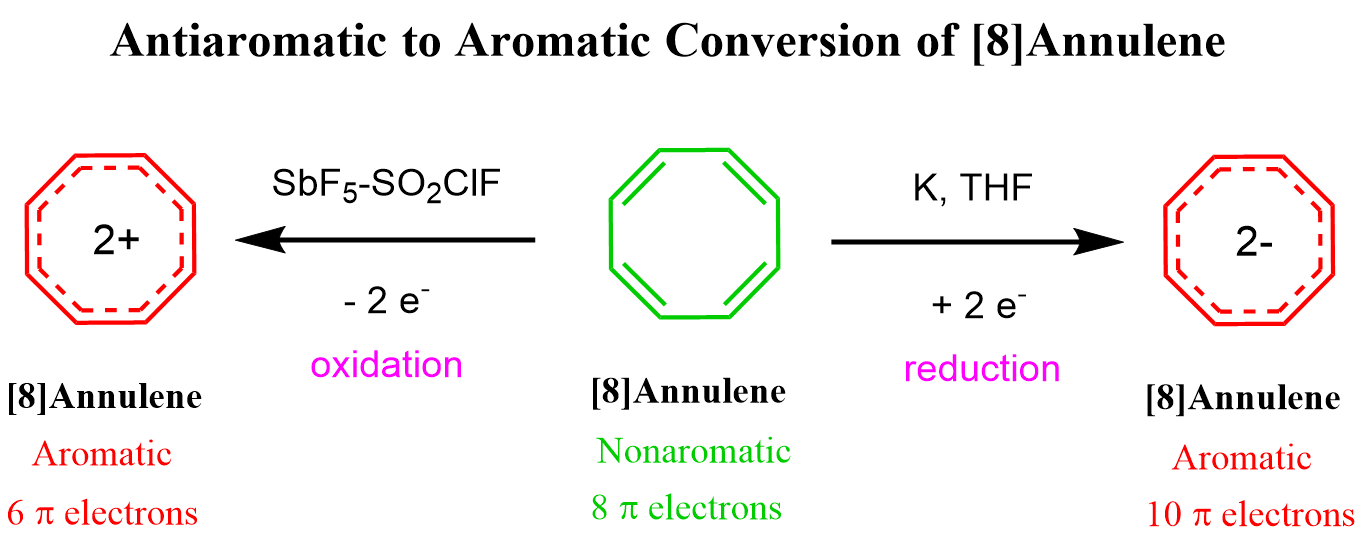
Now, since we mentioned Huckel’s rule and Frost circle, let’s how they are correlated.
Huckel’s Rule and Frost Circle
First, a quick reminder to distinguish aromatic and antiaromatic compounds. Aromatic and antiaromatic must be cyclic, planar, and fully conjugated, and the only difference is that aromatics have 4n + 2 π electrons, while antiaromatic have 4n π electrons:

As you will see throughout this article, Frost circle never contradicts Huckel’s definition, but none of them is an ultimate tool for distinguishing aromatic and antiaromatic compounds. We need also to consider the possibility of avoiding planar geometry, adopting a favorable hybridization, etc.
A good explanation of why there is a correlation between the Frost circles and Huckel’s rule is, as we will see later, the fact that there is always an odd number of bonding orbitals. What this does is one of the orbitals takes the two electrons from the 4n + 2 leaving 4n electrons which are equally paired in the remaining even number of bonding orbitals.

On the contrary, antiaromatic compounds (4n electrons), end up having unpaired electrons in the higher-energy orbitals after two electrons go to the bottom orbital. Remember, unpaired electrons are the main indicator of antiaromaticity in Frost circles.
Frost Circles for Three-Membered Rings
Because the Frost circle applies to fully conjugated compounds, we skip cyclopropene and focus on cyclopropenium cation and cyclopropenium anion. The cation has two π electrons which go to the bonding orbital leaving the antibonding orbitals empty. Therefore, cyclopropenium cation is aromatic.

There are two additional π electrons which go to the antibonding orbitals indicating an antiaromatic system. However, as we have already seen, the carbon atom may adopt sp3 hybridization to avoid antiaromaticity.
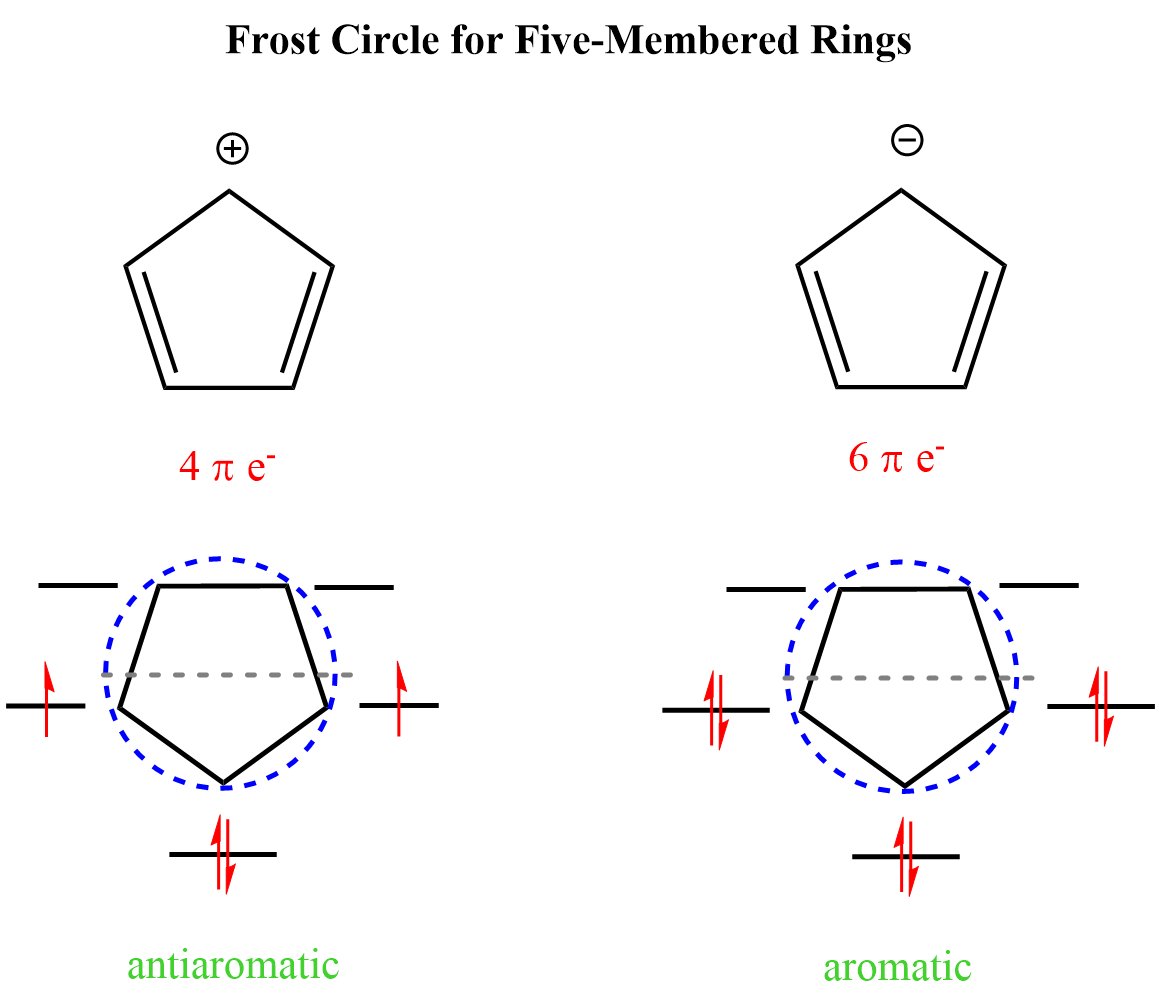
Frost Circles for Five-Membered Rings
Let’s compare the cyclopentadienyl anion and the cyclopentadienyl cation. There are three bonding orbitals for both. The cation is not aromatic because the bonding orbitals are not completely filled. In fact, it is antiaromatic because the bonding orbitals are not completely filled and are occupied by two unpaired electrons. On the other hand, the two extra π electrons in cyclopentadienyl anion are paired in the bonding orbitals making it antiaromatic:
Similar to the cyclopentadienyl anion, pyrrole, and furan, common and widely used molecules, are also aromatic as it has six π electrons in the conjugated system:
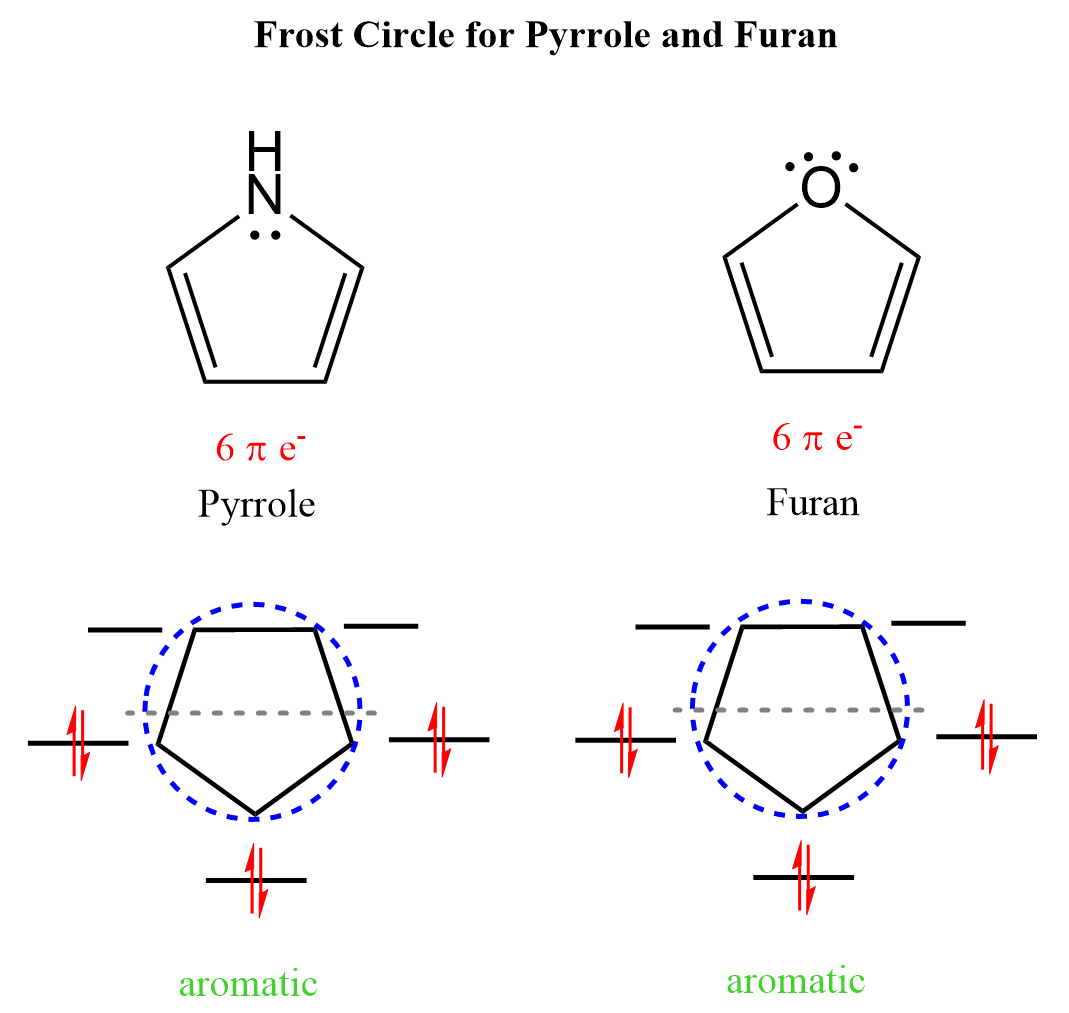
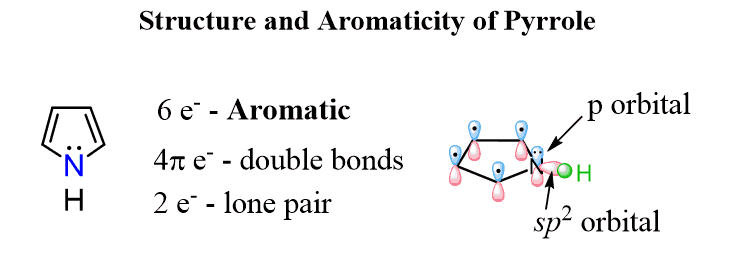
This prediction goes along with the Huckel’s rule. The nitrogen here looks to be sp3 based on the steric number (4 – 3 bonds and a lone pair) but because the molecule “wants” to be aromatic”, it changes the hybridization to sp2 thus placing the lone pair in a p orbital and forming a cyclic, planar, fully conjugated system of 6 electrons:
Notice that the other lone pair of the oxygen is not part of the aromatic system, and we ignore them when determining whether a molecule is aromatic or not. A similar situation occurs with another five-membered ring, imidazole containing two nitrogen atoms.
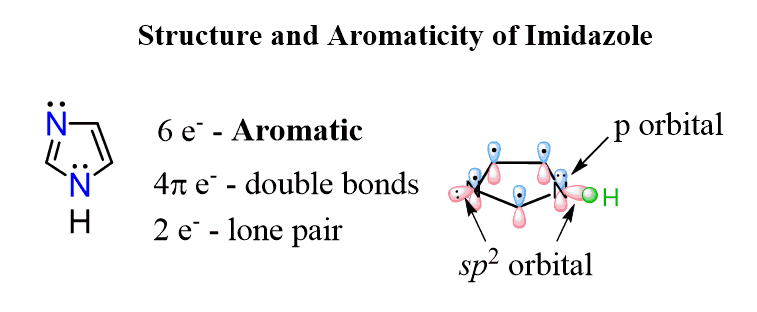
It has one lone pair in a perpendicular to the conjugated system sp2 orbital which is not part of the aromatic system and another one in a p orbital because the nitrogen with single bonds is sp2 hybridized favoring the aromaticity. This, in total, makes 6 electrons of the aromatic system and one lone pair which is not part of that. The Frost circle for imidazole will be identical to those of furan and pyrrole.
Frost Circles for Six-Membered Rings
Aside from benzene, pyrimidine, and especially pyridine, are also common aromatic compounds which we can confirm with the Frost circle method:
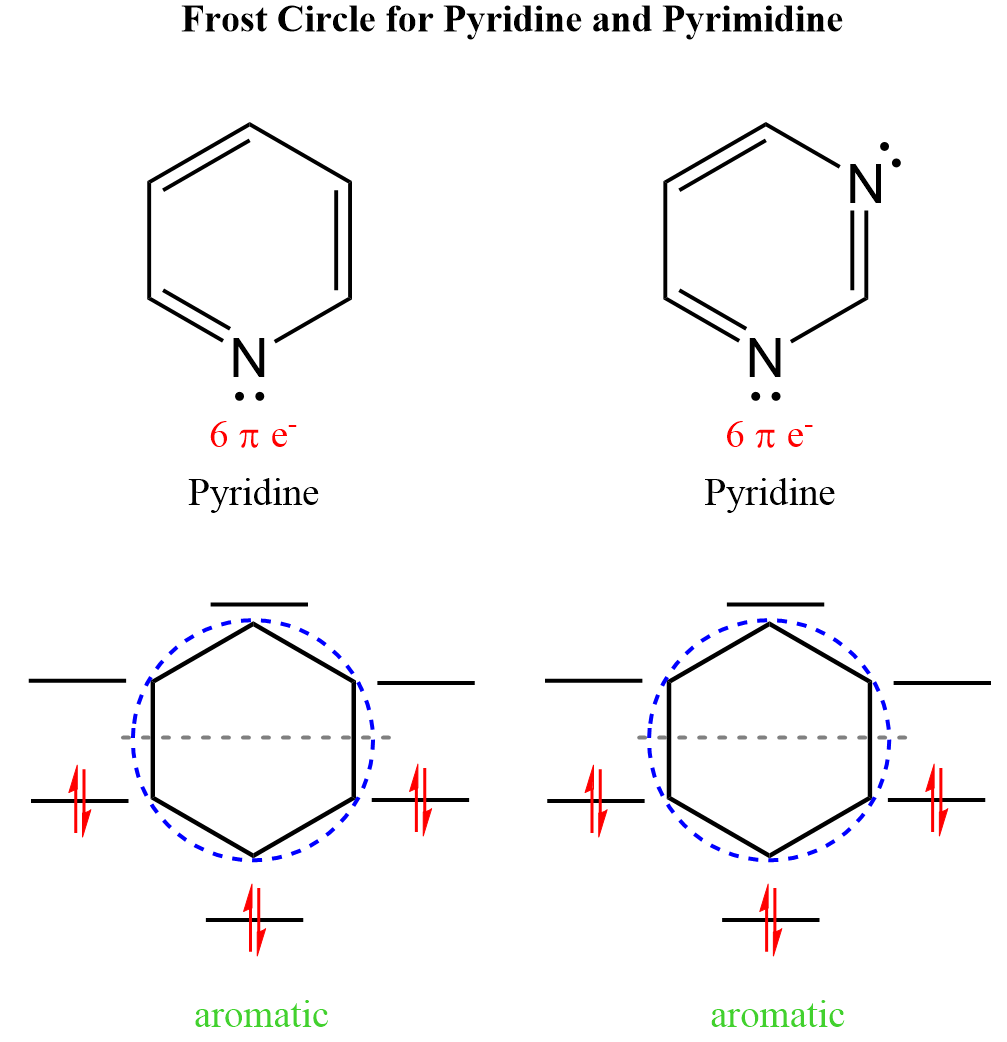
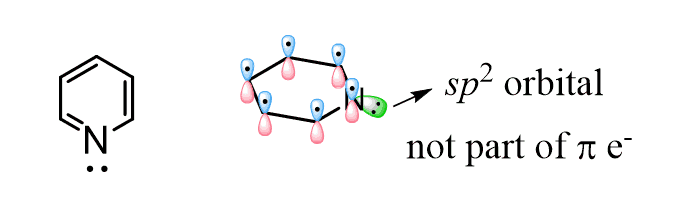
Notice that the nitrogen atoms are sp2-hybridized, and the lone pair is in an sp2 orbital of the nitrogen and thus not part of the conjugated system as the orbital is perpendicular to the p orbitals:
Frost Circles for Seven-Membered Rings
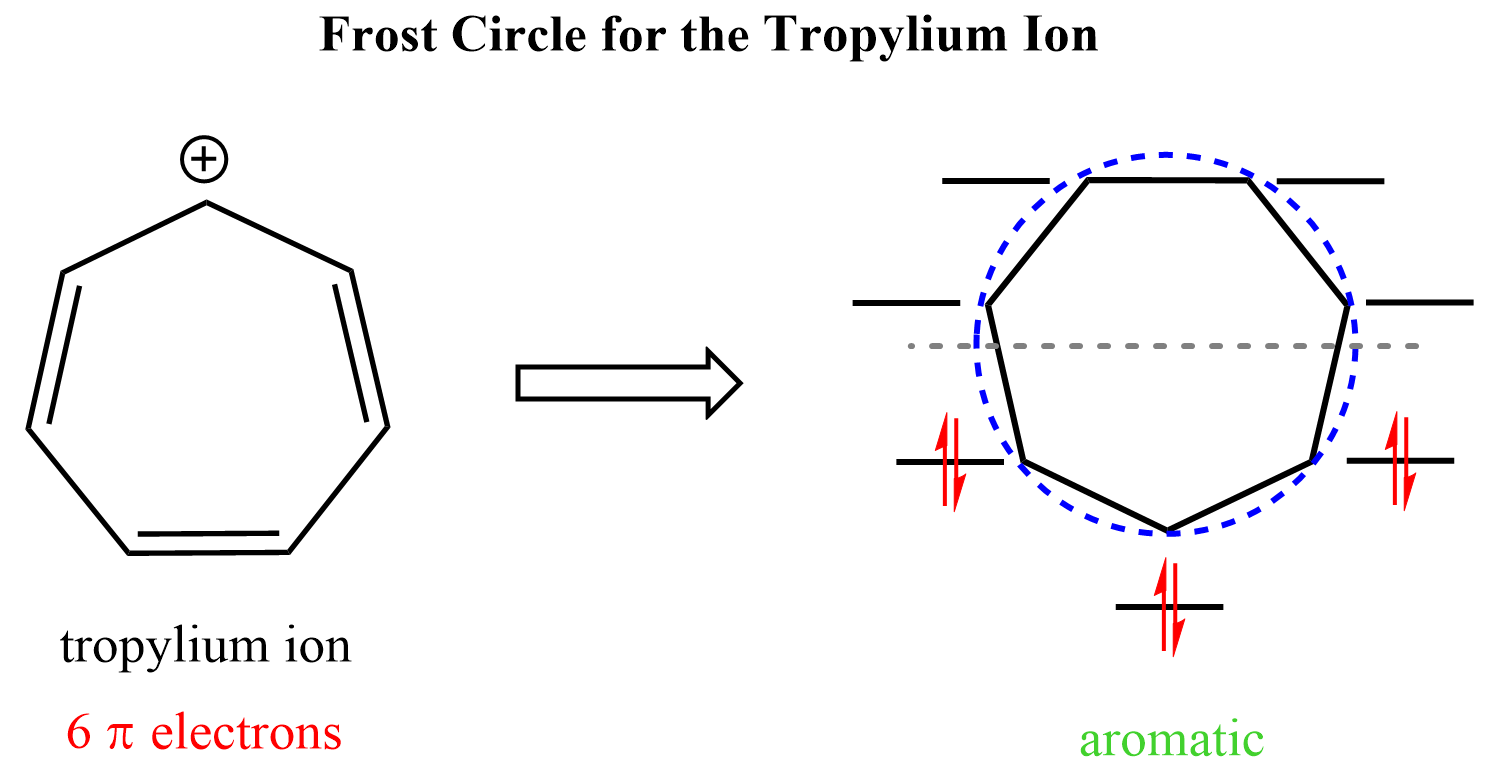
We cannot incorporate four double bonds in cycloheptane to obtain a fully conjugate system. Therefore, to be a candidate for aromatic or antiaromatic classification, a seven-membered ring must be either an ion or contain a heteroatom. The most common example in this category is the tropylium ion (cycloheptatrienyl cation) which is aromatic and quite stable:
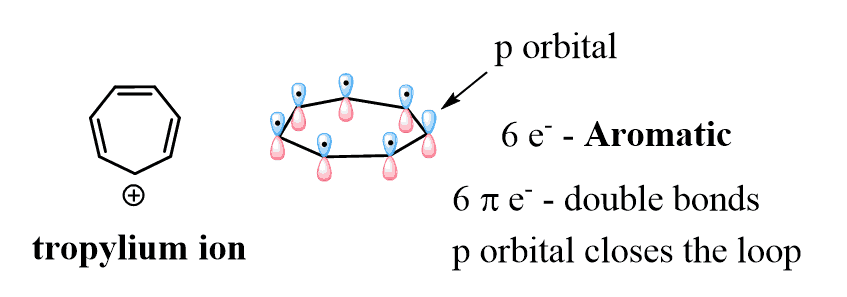
It has six electrons and they completely fill the three bonding orbitals leaving the higher energy ones empty:
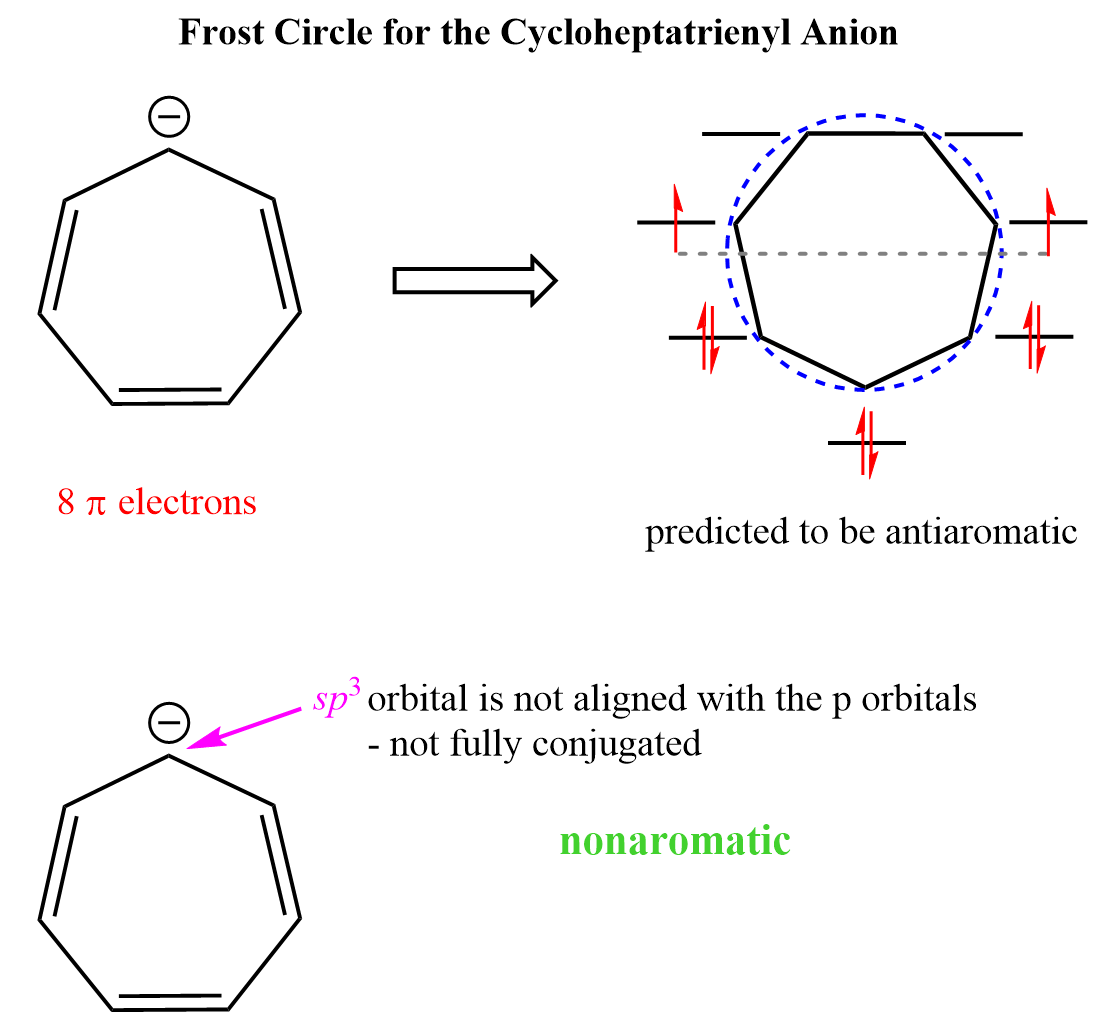
Just like cyclooctatetraene, the anionic analog of the tropylium ion, the cycloheptatrienyl anion is predicted to be antiaromatic based on the Frost circle, however, this time, the antiaromaticity is avoided by the anionic carbon being sp3-hybridized, thus removing the lone pairs from the conjugated system.
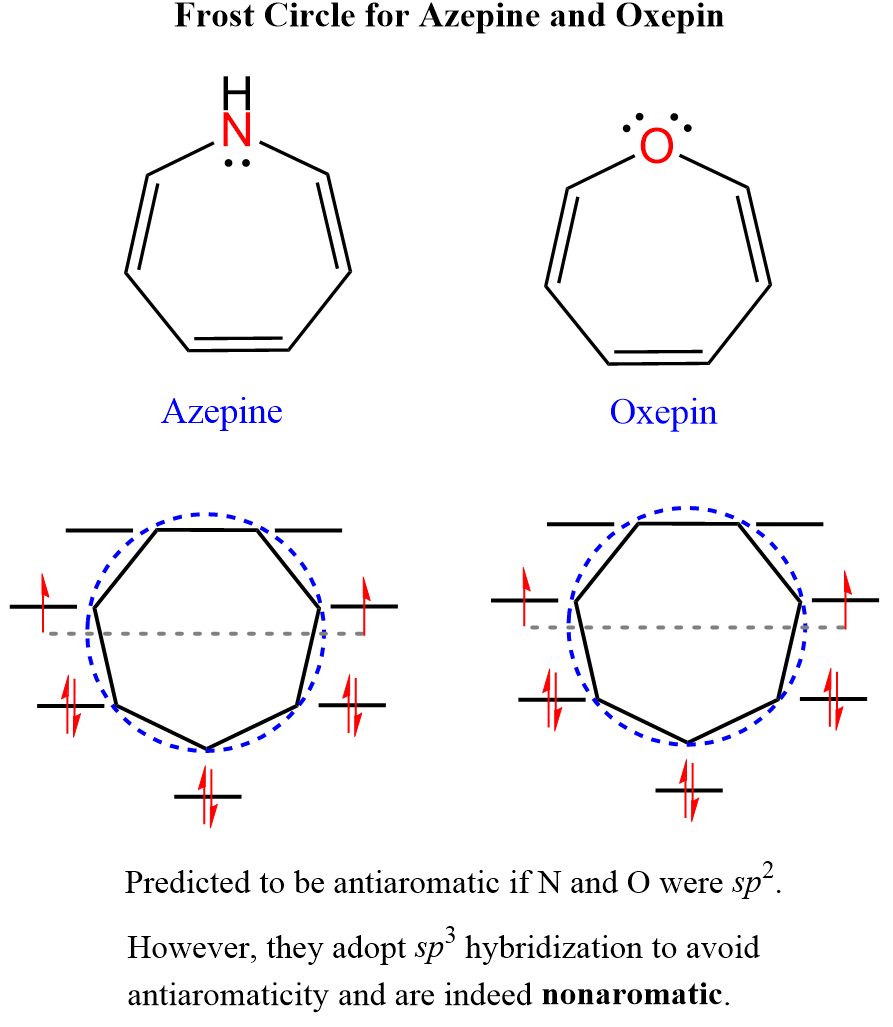
Another seven-membered ring is a candidate for an antiaromatic compound, because of the lone pair on the nitrogen, is azepine:
However, it is not a planar and 8π-antiaromatic compound as all the protons appear in the vinylic region which once again confirms that to be antiaromatic the molecule must not have another option. The oxygen analog of azepine is oxepin which is again not antiaromatic because the oxygen adopts and sp3 hybridization, as otherwise, being sp2 would make one of its lone pairs part of a fully conjugated cyclic system of 8 electrons making the molecule antiaromatic:
Frost Circles for Ten-Membered Rings
We have already discussed the Frost circle of cyclooctatetraene and discussed how it avoids being antiaromatic by adopting a nonplanar geometry. Cyclodecapentaene also known as 10[Annule] has 10 π electrons, and, according to Huckel’s rule, is predicted to be aromatic. However, because of the ring strain, it does not adopt a planar enough geometry to support the needed conjugation of the orbitals (Chemical Reviews, 2006, Vol. 106, No. 12 5347):

One way that has been successful in fixing the carbon atoms in one plane thus making the molecule aromatic, was by introducing a bridge such as a CH2 group – 1,6-methano-bridged [10]annulenes (J. Am. Chem. SOC. 1995,117, 1369-1373):

Notice that we called cyclodecapentaene [10]annulene, and this is a common name for cyclic ring systems especially for those with a greater number of atoms, but we will discuss them in the next article.
In summary, the Frost circle is a great method for determining whether a compound is aromatic or antiaromatic. Aromaticity is achieved when all the bonding orbitals are filled with paired π electrons, while no unpaired electrons are in the nonbonding and antibonding orbitals. The key to distinguishing these is the presence of unpaired electrons. Like Huckel’s rule, aside from the number and distribution of the electrons, we should also consider other factors such as geometry and unusual hybridization of atoms.
Check Also
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Aromaticity and Huckel’s Rule - Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta in EAS with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators ?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
