The alkylation of ketones is achieved through enolate intermediates which are formed by deprotonating the alpha position (the carbon next to the carbonyl group):
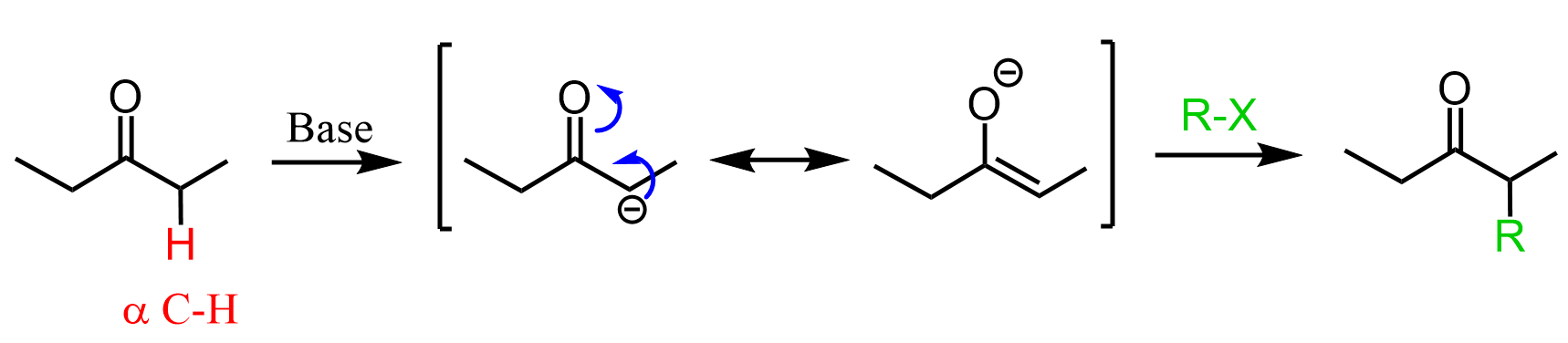
If you are still in the chapter on aldehydes and ketones, this may look foreign to you since all the reactions you have seen so far were directed to the C=O electrophilic carbon. So, yes, this is different because it starts with an acid-base reaction rather than a nucleophilic attack on the carbonyl. The acidity of the alpha hydrogen is very important and it opens a whole new chapter of Alpha Carbon Chemistry.
The enolate intermediate is very nucleophilic and undergoes a variety of reactions including alkylations:
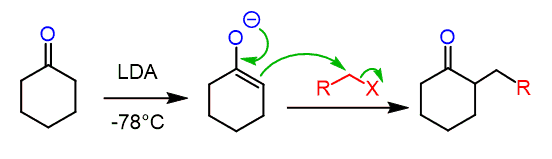
Like any other SN2 reaction, the alkylation of enolates works best with 1o, 1o benzylic, and 1o allylic substrates. Secondary and tertiary substrates would mostly undergo an E2 elimination forming an alkene.
The alkylation works also with other functional groups such as esters, nitriles, and nitro compounds since they also have an acidic ɑ position:
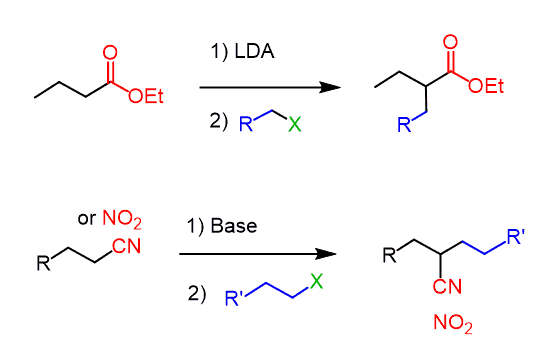
Aldehydes, on the other hand, are problematic for alkylation because of the competing aldol condensation.
The Regiochemistry of Enolate Alkylation
Unsymmetrical ketones can be alkylated on both sides depending on the base and the temperature. Alkylation of the less substituted carbon is achieved by using a sterically hindered base, and LDA is, by far, the most common base you are going to see being used for this purpose. The more substituted carbon is usually deprotonated by sodium hydride:
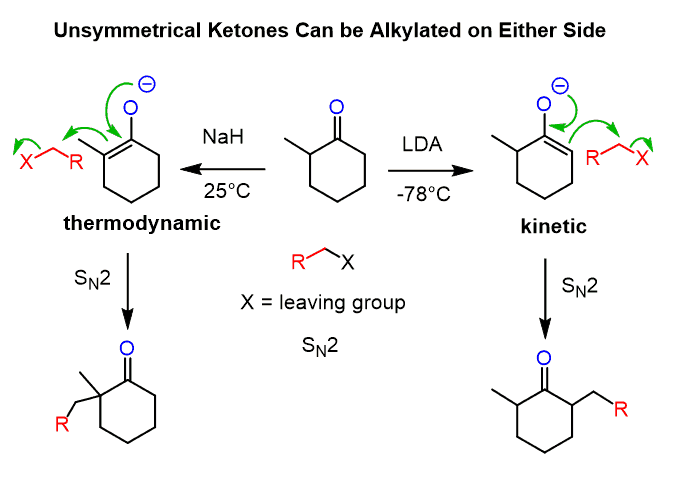
The less substituted enolate is the kinetic product (red pathway below) as seen from the lower activation energy (Ea) and therefore occurs faster.
The thermodynamic enolate (blue pathway) is the more stable enolate because of the more substituted C=C double bond.
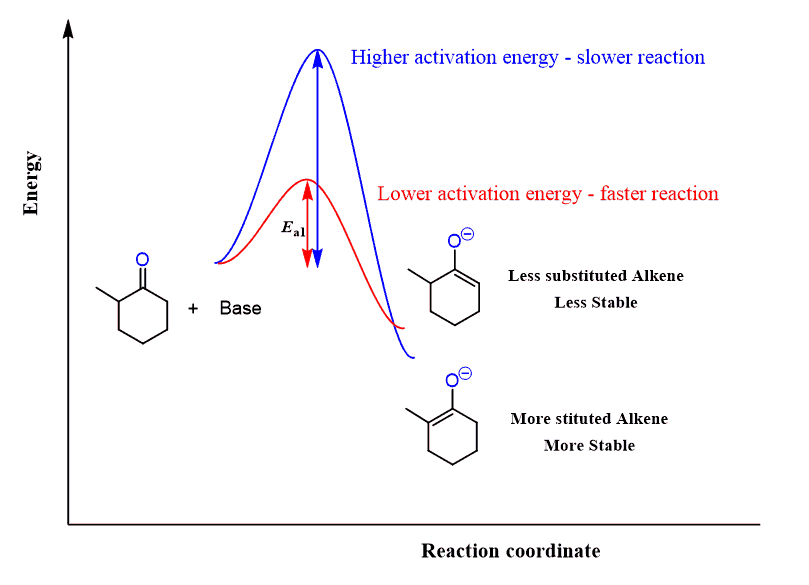
For a symmetrical ketone, it wouldn’t matter which of these bases is used.
The only thing is to make sure a strong base is used. Sodium hydroxide and ethoxides don’t work here because the enolate is not formed irreversibly and self-condensation reactions can occur because there is still a lot of carbonyl present in the equilibrium mixture. This is especially true for aldehydes which tend to undergo aldol condensation a lot faster than ketones do.
And, as always, if you are ready to test your skills, here is a good set of comprehensive problems on alpha carbon chemistry and also a separate one for the alkylation of enolates:
Enolate Alkylation Practice Problems
Enolates in Organic Synthesis – a Comprehensive Practice Problem
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
