Dehydration of alcohols using phosphorus oxychloride (POCl3) and pyridine (an amine base) in place of H2SO4 or TsOH is a good alternative for converting alcohols to alkenes when working with compounds that decompose in the presence of strong acids:

Let’s compare the mechanisms of acid-catalyzed dehydration and the elimination using POCl3. In both reactions, the OH needs to be converted into a good leaving group, and POCl3 helps in this by converting it into –OPOCl2, much like the strong acid does in acid-catalyzed dehydration:

Once the hydroxyl is converted into a good leaving group, pyridine removes a β-proton, which provides the electrons for making the C=C π bond.
The POCl3 elimination works for primary, secondary, and tertiary alcohols.
Notice that the elimination involves the base and the substrate, and all the bonds are being broken and formed at the same time.
You may recognize that this is an E2 mechanism, which is another advantage of using POCl3 over acid-catalyzed dehydration.
What do you think this advantage might be?
Remember, bimolecular reactions such as E2 and SN2 are always preferred since they do not form carbocations, which means no rearrangements can occur.
For example, using sulfuric acid for the dehydration of the following alcohol results in a rearrangement forming an alkene that might not have been the desired product:

However, POCl3 prevents this, and the major product of the reaction is the alkene that is expected according to the Zaitsev’s rule.
The elimination of alcohols can also be achieved by converting the alcohol into an alkyl halide using either a hydrohalic acid (HCl, HBr, and HI), or SOCl2 or PBr3, and reacting it with a strong non-hindered base:
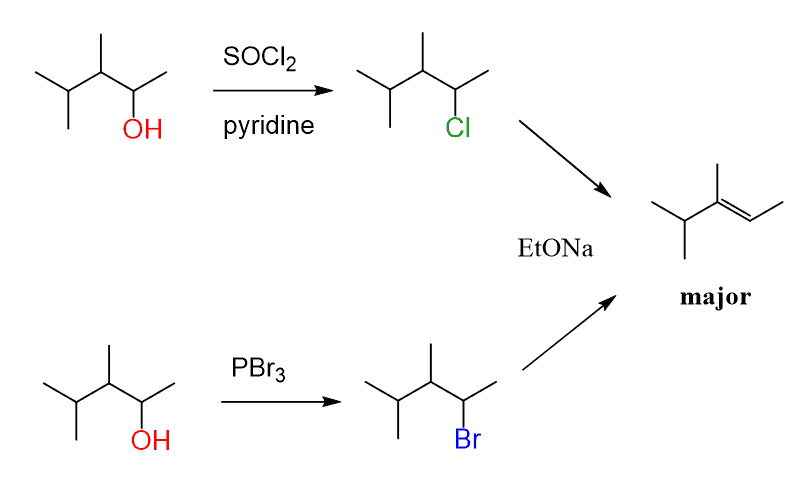
One advantage of the POCl3 compared to these methods is the saving of time since the transformation is achieved in one step.
If the Hofmann product is the desired alkene, the alcohol can first be converted into a mesylate or tosylate, then treated with a strong, bulky base such as potassium tert-butoxide (tBuOK) to drive the elimination.
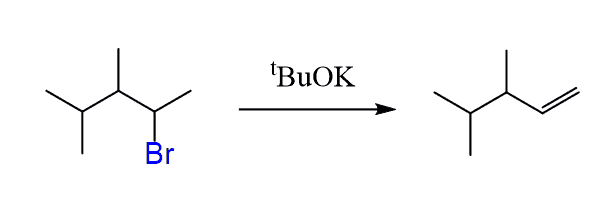
In summary, POCl3 works for most alcohols, allows to carry out the reaction in mild conditions, and no rearrangements occur since the reaction goes by the E2 mechanism according to Zaitsev’s rule. A great technique!
Check Also
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols



hi, in the POCl3 alcohol elimination, how can pyridine deprotonate the compound given? i looked up the pKa value for pyridine, it was 5.2.
what am I missing here?
Yes, the pKa of the conjugate acid of pyridine is about 5.2, and for the deprotonation step, we are comparing this value with the pKa of the conjugate acid of an alcohol or an ether. These are around the negative 2-4 region and the reaction is expected to work since the pKa goes higher (-3 to 5.2). Remember, any acid-base reaction goes towards the formation of a weaker acid (higher pKa).
Here is a link you can follow to read about it more:
How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound
how pocl3 will react with dihydroxy diphenol compound in presence of Pyridine?
Can any one guide?