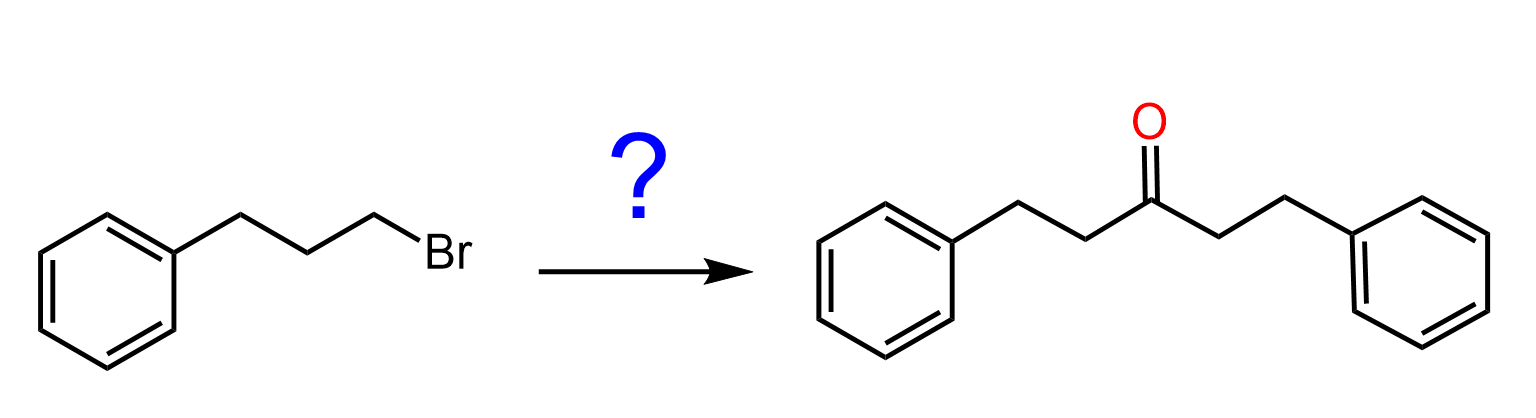
Acyl chlorides are very useful in reactions with organometallics because they can selectively be converted into alcohols or ketones.
For example, Grignard reagents will react with acyl chlorides to produce alcohols, while organocuprates (Gilman’s), also known as lithium dialkyl cuprates, can be used for making ketones from acyl chlorides:
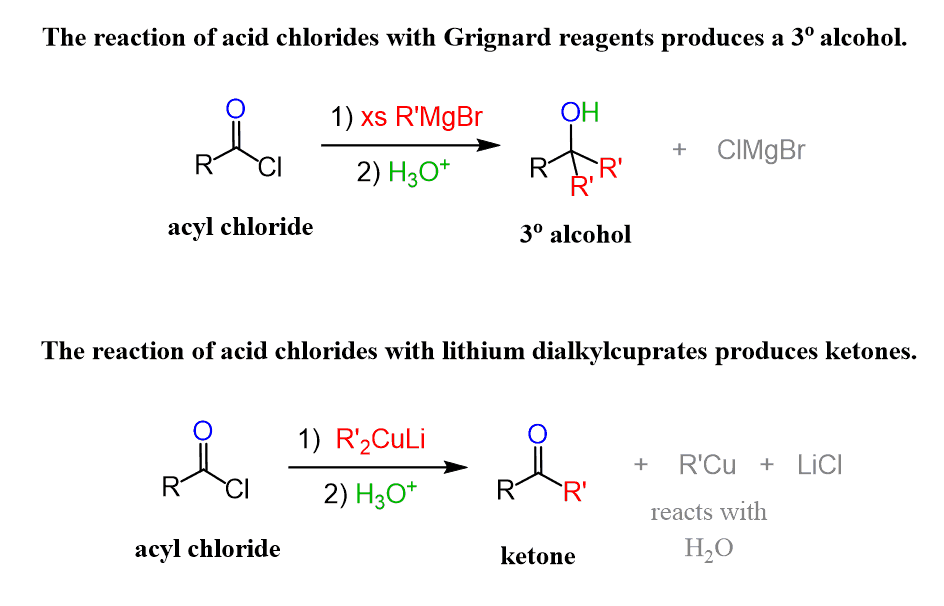
So, the question is – how come the Grignard and Gilman reagents react differently with acid chloride?
The short answer is that the Gilman reagent is less reactive than Grignard because the alkyl groups are connected to copper rather than magnesium. This makes their carbanionic character less pronounced since the C−Cu bond is less polarized than a C−Mg bond.
Let’s also draw the mechanism of these reactions to better understand this selectivity.
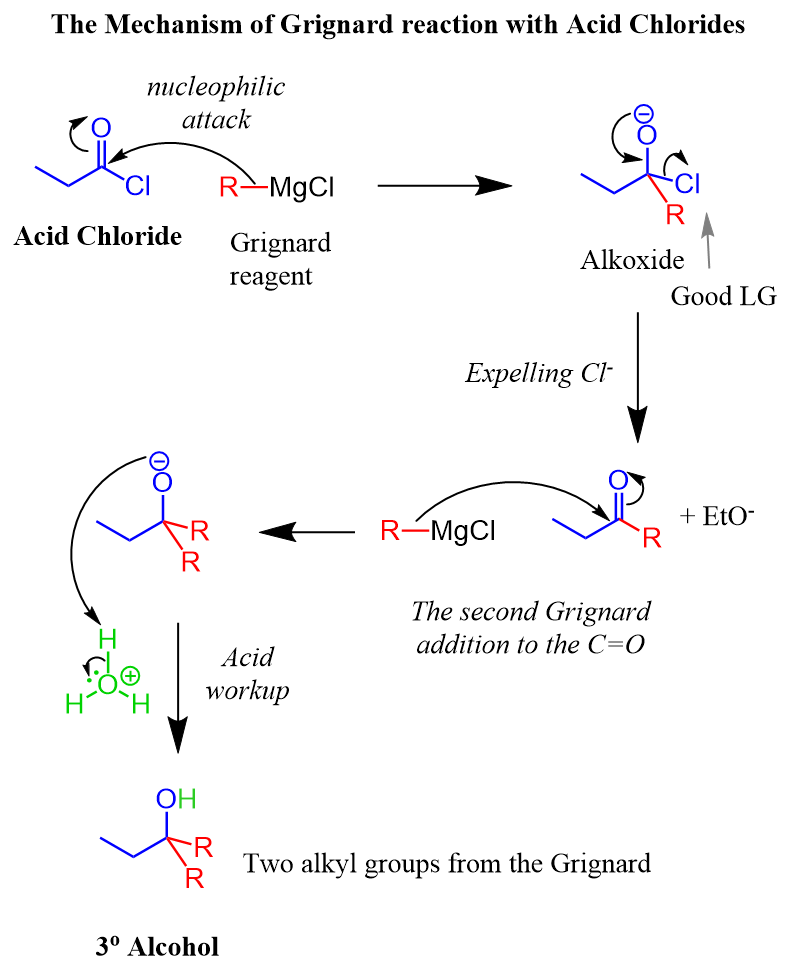
The mechanism of nucleophilic acyl substitution for acid chlorides is similar to the one for esters.
Remember, esters require an excess of Grignard reagent because there is a ketone formed after the first reaction, and the ketone is then converted into an alcohol by the second equivalent of the Grignard reagent:

If one equivalent of the Grignard reagent is used, a mixture of starting material, ketone, and alcohol is formed. Check this post for more details.
The same thing happens when an acyl chloride reacts with a Grignard. For the mechanism, you only need to replace the OR in the ester with a chloride:
Now, organocuprates react just like the Grignard, except the reaction stops once the ketone is formed because Gilman reagents do not react or react very slowly with ketones!

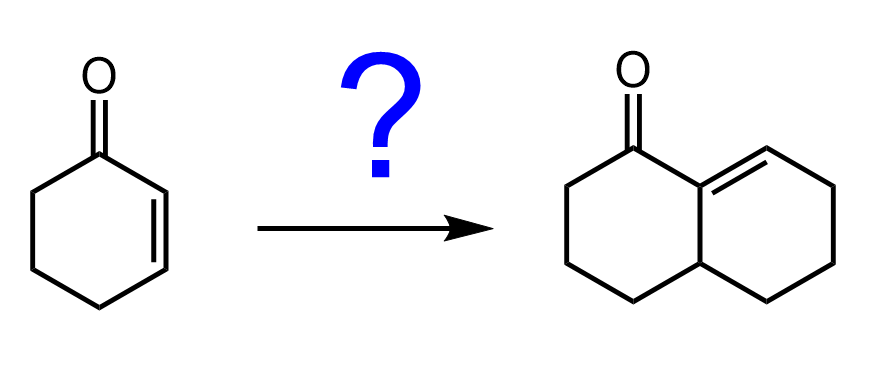
I do want to mention that organocuprates react with ɑ, β-unsaturated ketones, possibly because of making a π complex with the double bond.
The reaction, however, is slightly different as the alkyl groups add to the carbon of the double bond rather than to the one of the carbonyl:

This is called 1,4-addition, unlike, for example, the Grignard reaction, which is a classical 1,2-addition. This has to do with the “hard” and “soft” acids and bases concept, and we will dedicate a separate post about this and the reactions of organocuprates in general.
💥 Check out this comprehensive review of the reaction of Grignard (RMgX), Organolithium (RLi), and Gilman (R2CuLi) Reagents to understand how they differ in their addition to carbonyl compounds and other electrophilic species.
One more thing – the mechanism of organocuprates is a controversial subject, and even though we show it as a “nice” ionic reaction, keep in mind that they may be radical as well. I would say what we showed here is likely an acceptable mechanism for undergraduate courses especially. However, ask your instructor how to go about it.
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- The Addition-Elimination Mechanism
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carboxylic Acids and Their Derivatives
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives

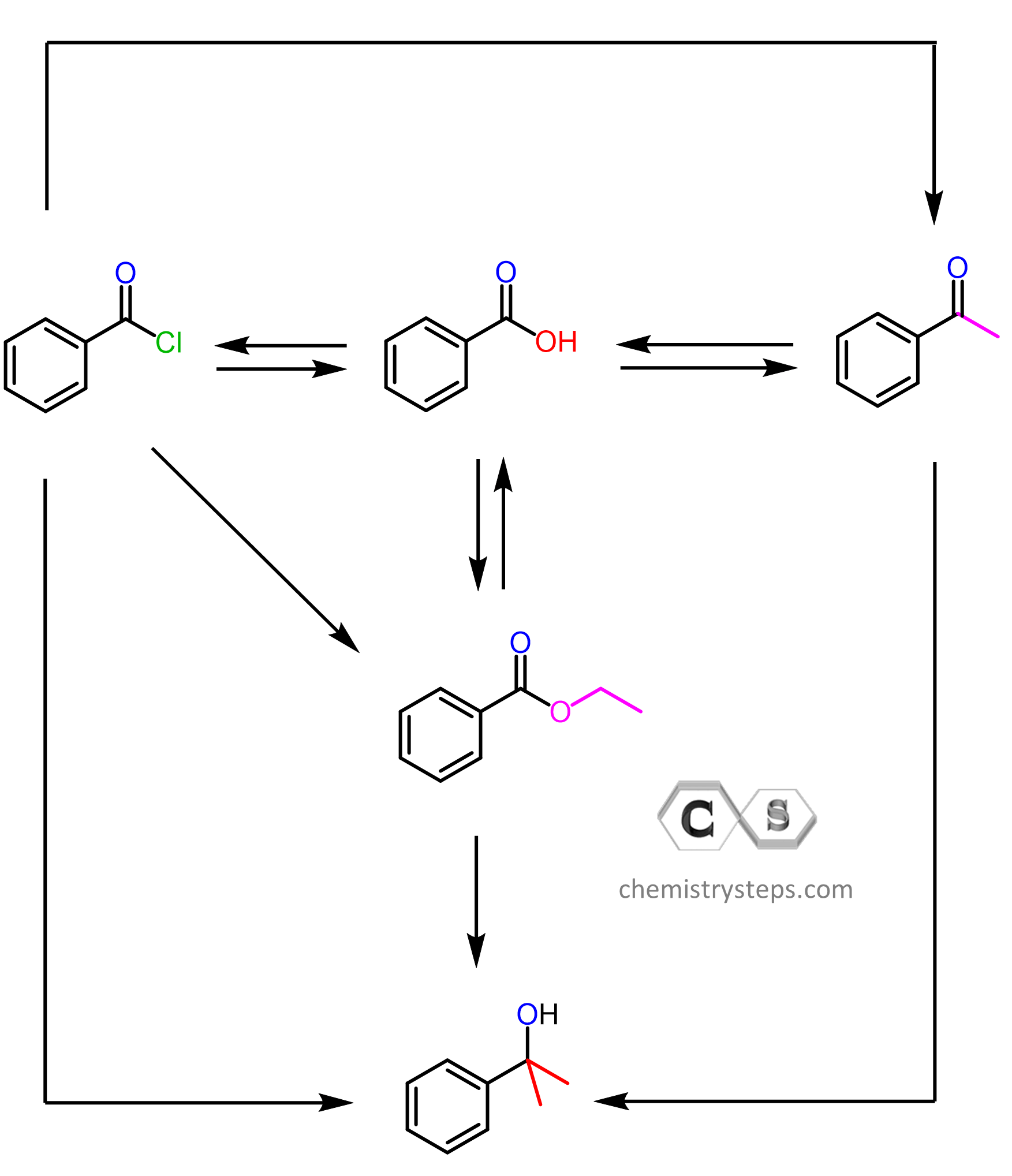
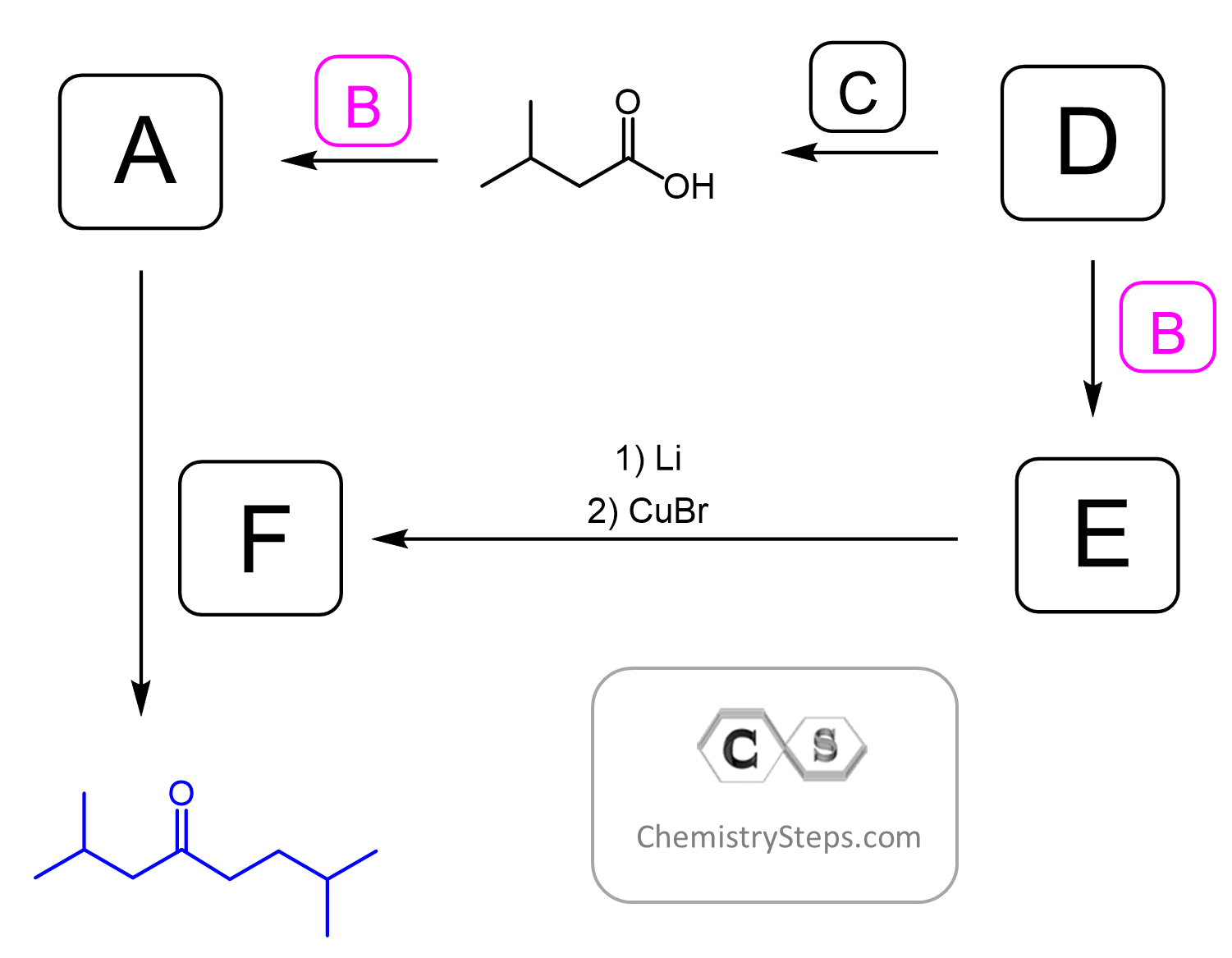

Does the reaction of the Gilman reagent (and Grignard) only work with acid chlorides or can it be any halogen instead of the chloride as well?
And I am also confused about the alkyl groups on the left side of the carbonyl group in figures 2, 3 and 4. (In the beginning it is ethyl but after the first step it is isopropyl.)
Thanks in advance!
Hello, and thanks for letting me know about the images!
Now, for the acyl halides, yes, all of them – acyl chlorides, bromide, iodides, and fluorides can be prepared and most of them are stable compounds. For example, acyl bromides (RCOBr) can be prepared with similar reagents as the chlorides, which would mainly be SOBr2 and PBr3.
For the reactions of these derivatives, you need to keep in mind that the main reason for using the chlorides is their affordability compared to the other analogs. Making acyl bromides is a more expensive process and they are rather used as intermediates in the alpha bromination of carboxylic acids.
Acyl fluorides and iodides, on the other hand, are prepared from acyl chlorides or anhydrides, and this alone makes them of little use, even though there are some exceptions. You can google the use of acetyl iodide. Also, the acyl fluorides are generally a lot less reactive than the chlorides because of the better orbital overlap between the F and C atoms in the resonance hybrid of the acyl fluoride as O, C, and F are all in the same row of the periodic table and thus have comparable sizes of p orbitals (recall the reactivity of amides vs esters for example).
A better orbital overlap means better electron donation from the halogen to the carbon which means less partial positive charge on the carbon (less electrophilic) and therefore, not as reactive towards nucleophilic addition/substitution reactions.
Again super amazed by your detailed answer, thank you!
You are most welcome!
How about in the case of aldehydes? Does gilman do the same as acyl chlorides or does it undergo 1,4-addition? Thank you
a,b-unsaturated carbonyl compounds can undergo 1,4-addition with organocuprates, while regular aldehydes and ketones are generally unreactive to them.