Preparation of Phenols
Phenols can be prepared industrially by a substitution reaction of aryl halides under extreme conditions:
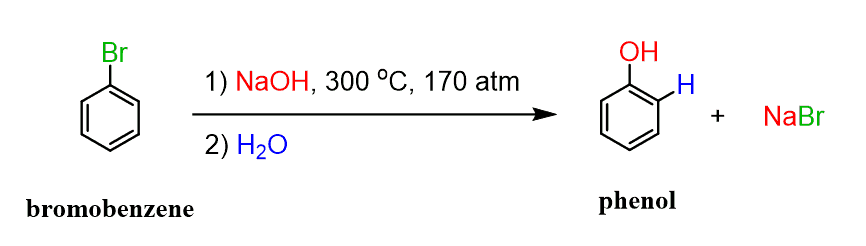
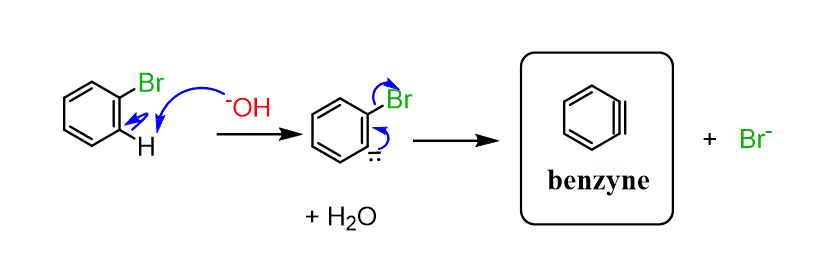
This is a type of nucleophilic aromatic substitution via a benzyne intermediate:
Milder conditions suitable for laboratory preparation of phenols are possible if there is an electron-withdrawing group on the ring that goes through the addition-elimination mechanism of nucleophilic aromatic substitution:
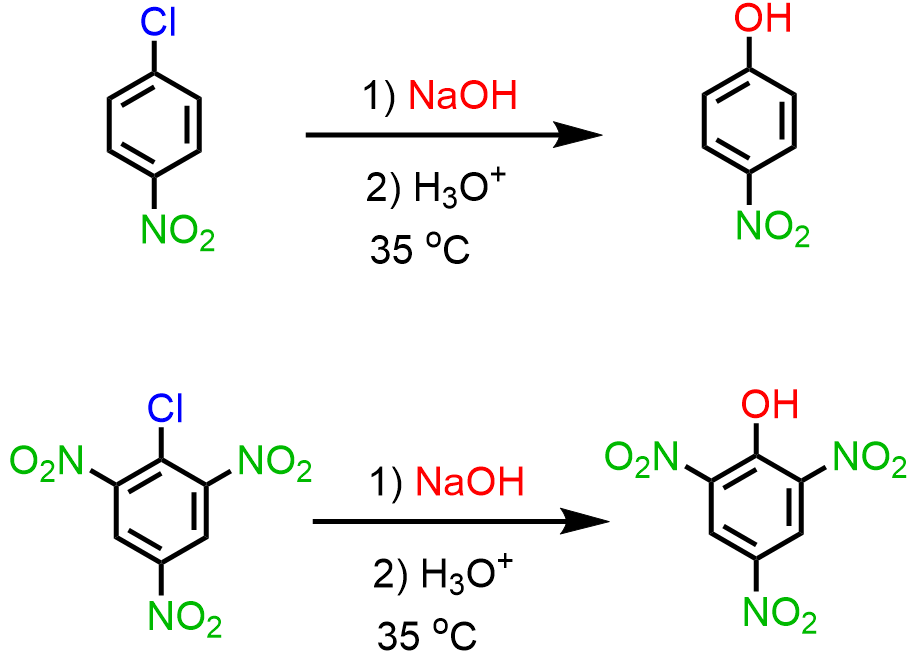
Notice that increasing the number of electron-withdrawing groups decreases the required temperature. You can read more about the mechanism and radiochemistry of nucleophilic aromatic substitution here.
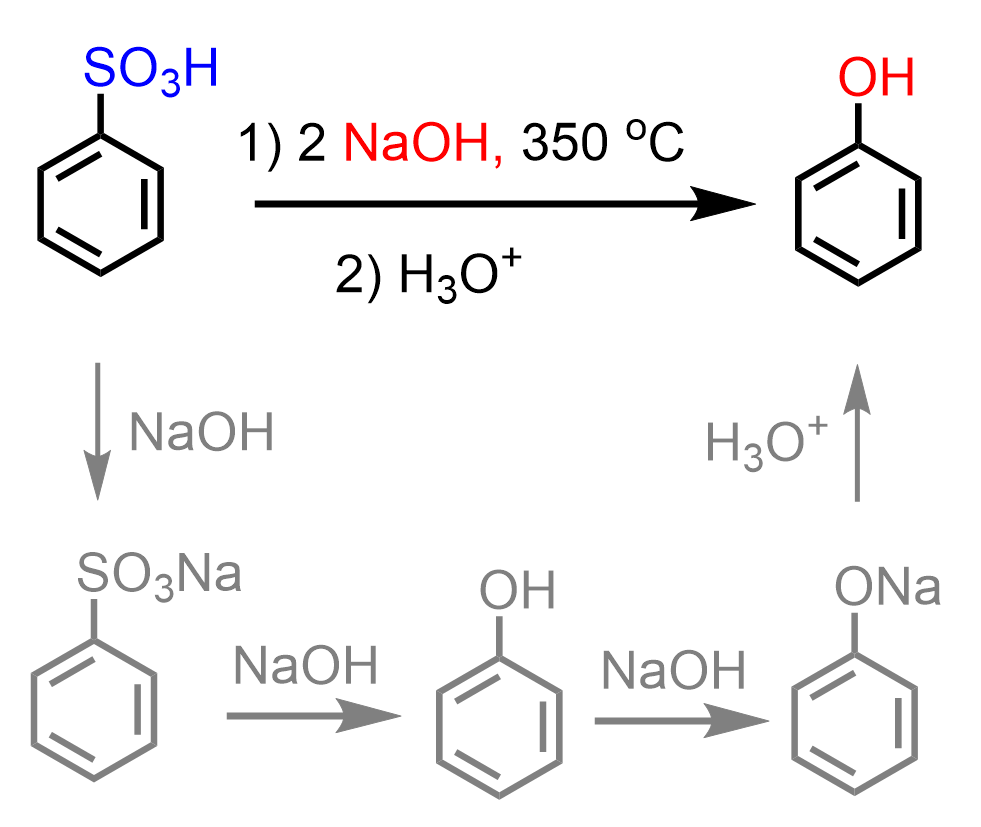
In a similar nucleophilic aromatic substitution, benzenesulfonic acid can be converted to phenol. The intermediates of this reaction are sodium benzenesulfonate and sodium phenoxide:
Another industrial method is the cumene (isopropylbenzene) process, where phenol and acetone are prepared from benzene and propylene via a mechanism involving radical intermediates.
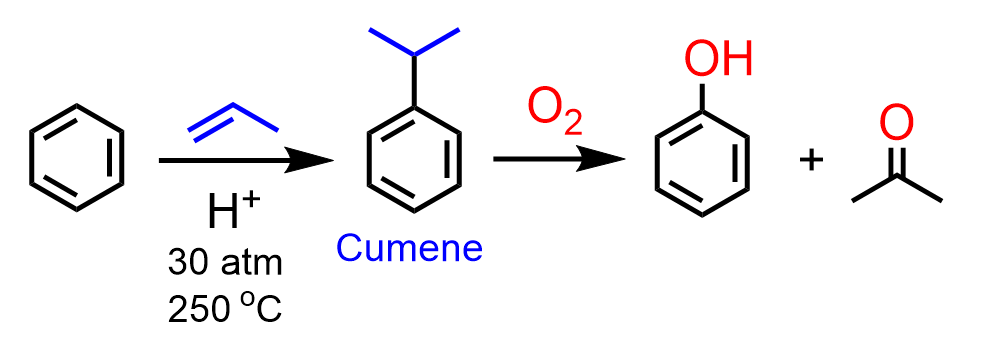
Notice that the first step is a Friedel-Crafts alkylation.
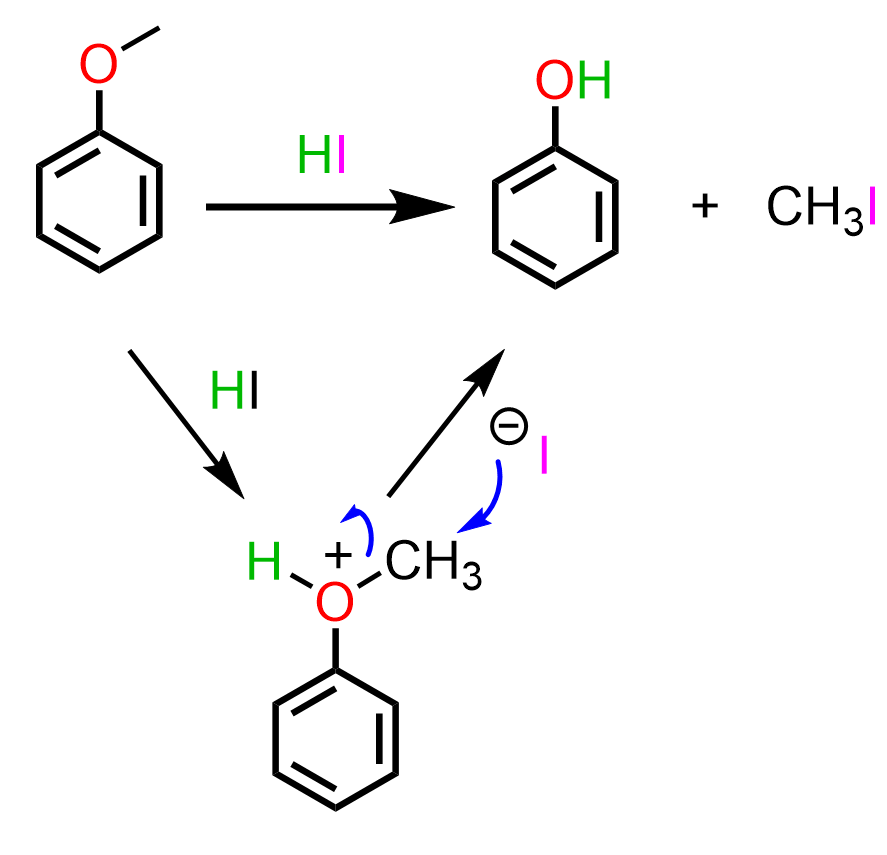
Anisole or other ether derivatives can be converted to phenol by acid-catalyzed cleavage:
Phenols can also be prepared from benzene derivatives by converting them to an arene diazonium salt and subsequently hydrolyzing them:
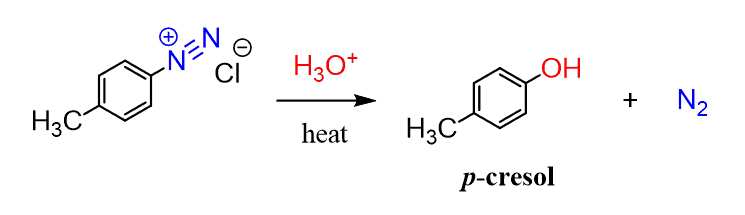
The addition of cuprous oxide can increase the yield of this reaction. Arenediazonium salts are excellent precursors in preparing various aromatic compounds, and you can read about them in this dedicated article.
Phenols in Electrophilic Aromatic Substitution
Phenols are great substrates for halogenation, nitration, sulfonation, and Friedel–Crafts reactions, as they often do not require a catalyst for electrophilic aromatic substitution. The OH group is an ortho, para director, and because of the strongly activating feature of the OH group:
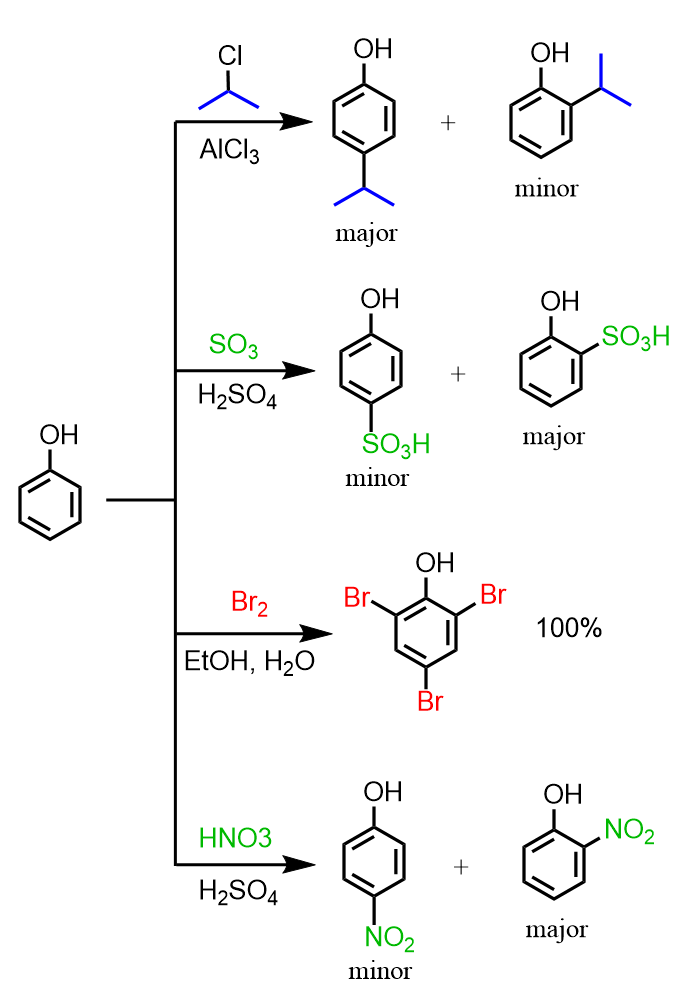
The ratio of the ortho and para substitution is difficult to predict. On one hand, the para position is favored because of the steric hindrance of the ortho position; on the other hand, there are two ortho positions and only one para, which statistically increases the yield of the ortho substitution. The larger the group present on the ring, the more favored the para substitution would be.
As we can see from the sulfonylation nitration of phenol, the intermolecular interaction, such as hydrogen bonding between the OH and the other group, also contributes to the formation of the ortho isomer. Check the post on ortho, meta, and para substitution for more details and examples.
Notice that for the nitration of phenol, dilute nitric acid is used to avoid undesired oxidation reactions.
Notice also that Cl2 and Br2 react with phenol and other benzene derivatives with strongly activating groups, such as NH2, without a catalyst, and transubstantiation occurs. With nonactivated or deactivated aromatic compounds, the halogens require the presence of a Lewis acid catalyst (AlCl3 or FeBr3).
A monobromination at the para position can be achieved by using one equivalent of Br2 at lower temperatures (< 5 °C):
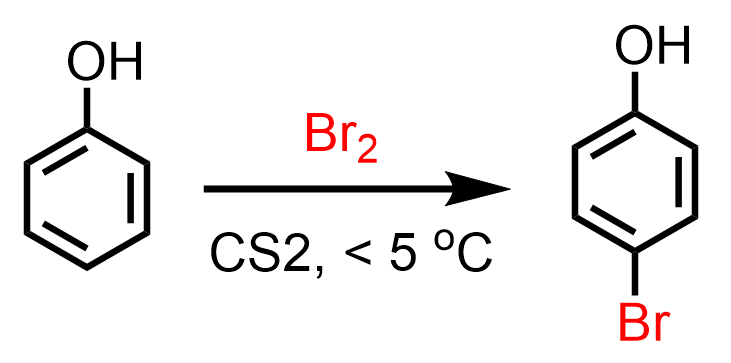
Iodination of phenol and other benzene derivatives is more difficult because it is a very weak electrophile, and only reacts in the presence of silver(I) salts, HNO3, or other oxidants. N-iodosuccinimide (NIS) and N-bromosuccinimide (NBS) have also been shown to work for the iodination and bromination of benzene derivatives. The reagent ICl is a better iodinating agent than molecular iodine. Fluorine, on the other hand, is too reactive, and the reaction is vigorous with no practical use in laboratories. You can find more about the halogenation of arenes in March’s Advanced Organic Chemistry.
Why is OH a Strongly Activating Group?
Due to the strong activating power, anisole undergoes nitration about 10,000 times faster than benzene and about 400 times faster than toluene. All the other electrophilic aromatic substituting reactions are also faster when oxygen or nitrogen is connected to the aromatic ring. Now, you may wonder why it is the case if they are more electronegative than carbon and should pull the electron density from the ring, thus making it less reactive. Recall that the reason for such a strong activating power of the oxygen and nitrogen is the resonance stabilization of the cation by the nonbonding electrons, which other activating groups such as alkyls do not have:
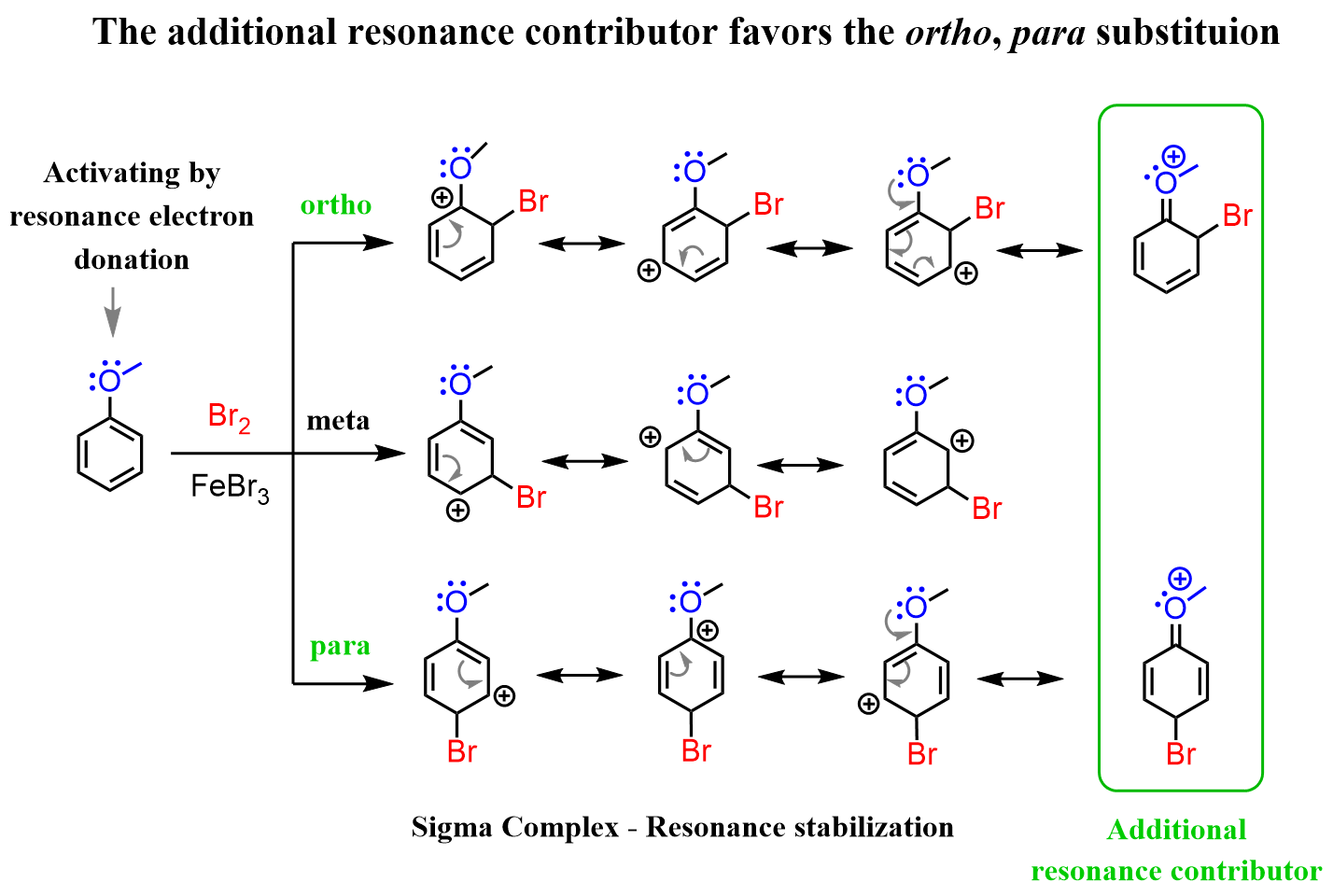
So, even though oxygen pulls the electron density from the ring via the inductive effect, the +M mesomeric (electron-donating resonance) effect is more profound, which makes it a strongly activating group.
Friedel-Crafts Reactions of Phenols
Although phenols are Lewis bases, and the oxygen does bind to the Lewis acid catalyst, and the reactions may require higher temperatures, the Friedel-Crafts reactions are still possible, especially so for anisole, where the methyl group protects the oxygen from the Lewis acid.
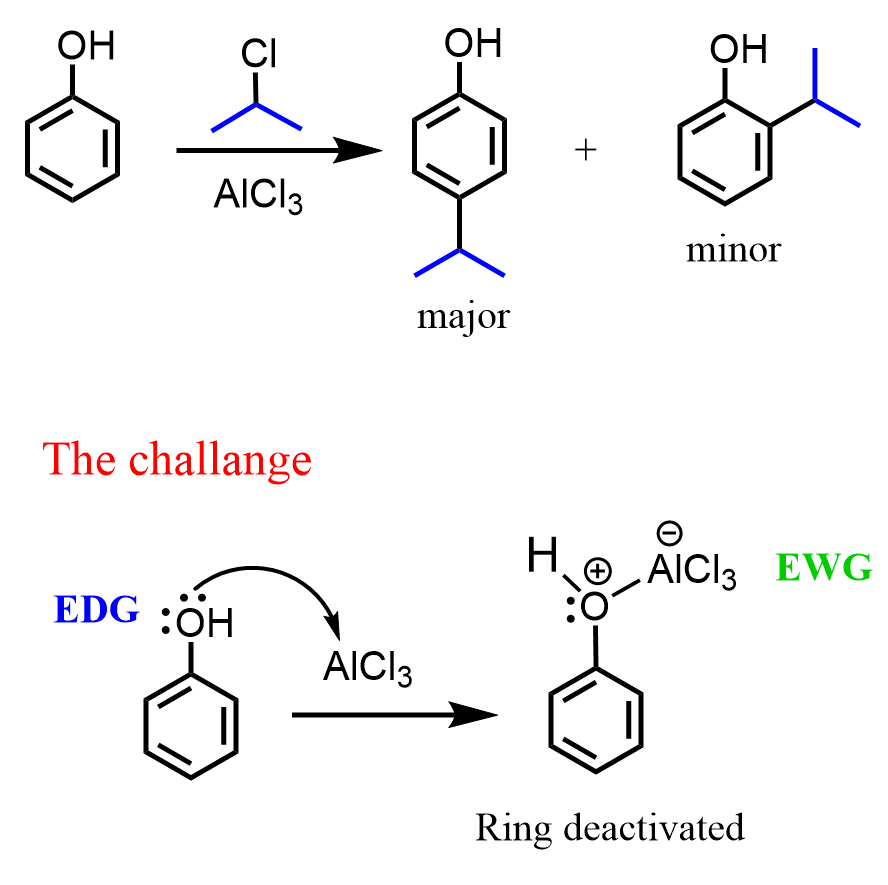
This is not true for aniline, because nitrogen is a stronger base and binds to the Lewis acid catalyst, making the ring completely unreactive.
Oxidation of Phenols
Phenols undergo oxidation in the presence of typical oxidizing agents such as chromic acid (Na2Cr2O7 is the precursor), forming 1,4-conjugated diketones called quinones:
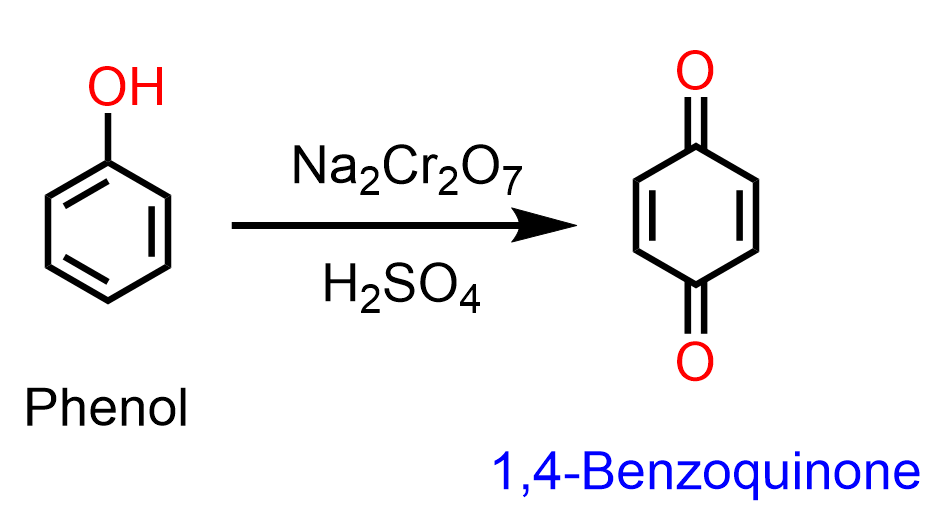
Oxidation of hydroquinone (1,4-benzenediol) occurs even with milder oxidizing agents such as AgBr:
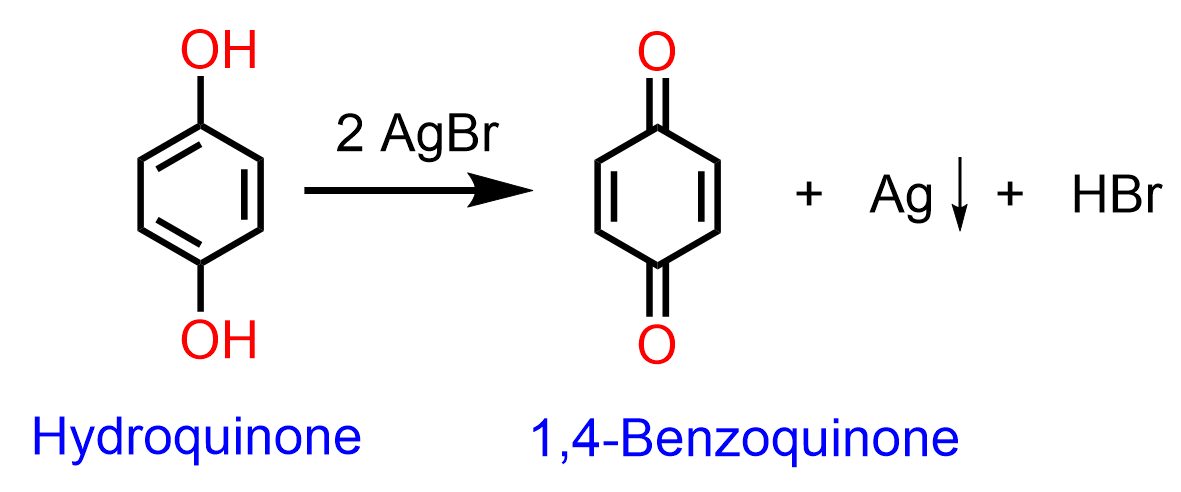
The oxidation of phenols to quinones is reversible and plays an important role in the biochemical process, as it serves as a transport for a pair of electrons from one substance to another in enzyme-catalyzed reactions.
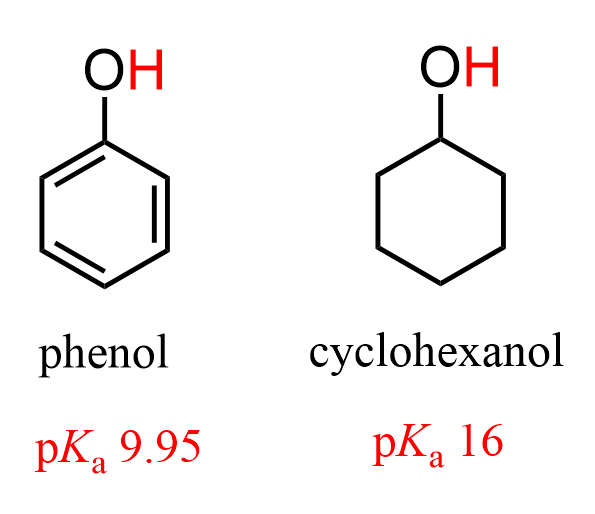
The Acidity of Phenols
Because of the resonance stabilization of its conjugate-base anion, phenols are a lot more acidic than aliphatic alcohols:
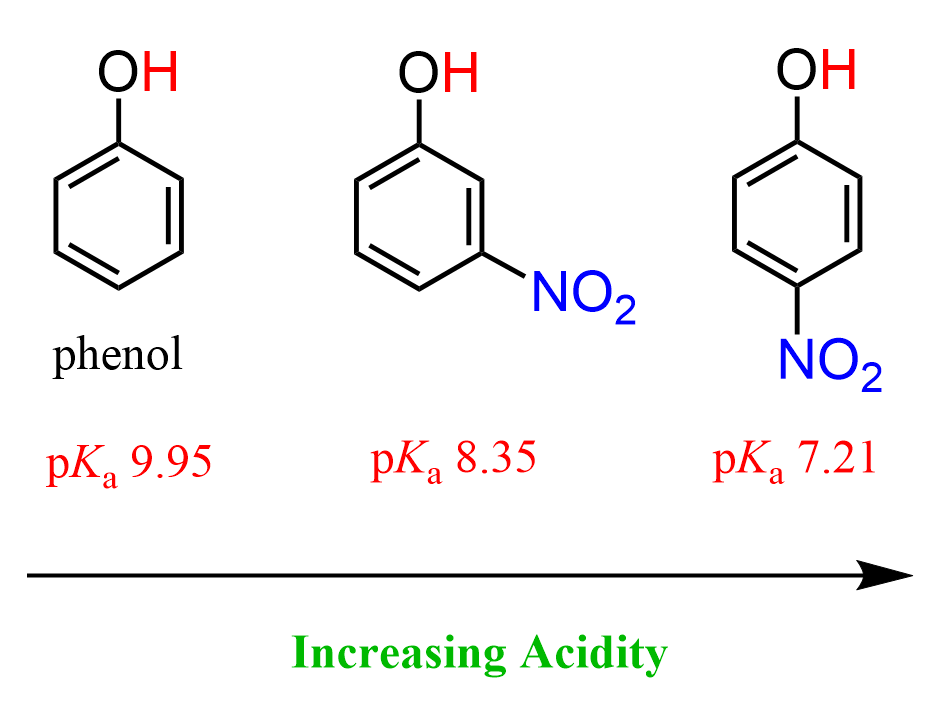
The presence of electron-withdrawing groups (EWG) increases the acidity even more a they help stabilize the negative charge of the phenoxide ions. As expected, having an electron-donating group on the ring reduces the acidity of phenol because it brings additional electron density rather than delocalizing it:

Aside from the nature of the group, we also need to pay attention to its position – the orientation of the group relative to the OH group. For example, substituent groups can also affect phenol acidity by both polar and resonance effects. For example, p-nitrophenol is more acidic than m-nitrophenol even though the p-nitro group is farther from the phenol oxygen:
This cannot be explained by the electronegativity of the nitro group since this effect gets smaller as the distance between the acidic proton decreases. And, as always, resonance stabilization comes to the rescue when explaining many features of organic molecules. When the nitro group is in the para position, it can participate in the delocalization of the lone pair on the nitrogen, while, in the meta position, it cannot:

A significant contribution of the highlighted resonance structure is that it places the negative charge on the more electronegative oxygen atoms, which is not the case for the m-nitrophenol:
Phenol as an Oxygen Nucleophile
The oxygen of the OH group in phenol is not as nucleophilic as it is in aliphatic alcohols because of the delocalization of the lone pairs and the steric effect of the aromatic ring; however, we can activate it by deprotonation to prepare a phenoxide ion, which then reacts with electrophiles such as alkyl halides, acyl halides, acid anhydrides, etc..

Due to the high reactivity of acid chlorides, they can also react with phenol.
Phenol in The Synthesis of Aspirin
The phenoxide ion also acts as a carbon nucleophile due to the resonance activation of the ring. For example, it is used as an industrial method for the preparation of salicylic acid (Kolbe-Schmidt reaction), which is then converted to aspirin (acetylsalicylic acid):
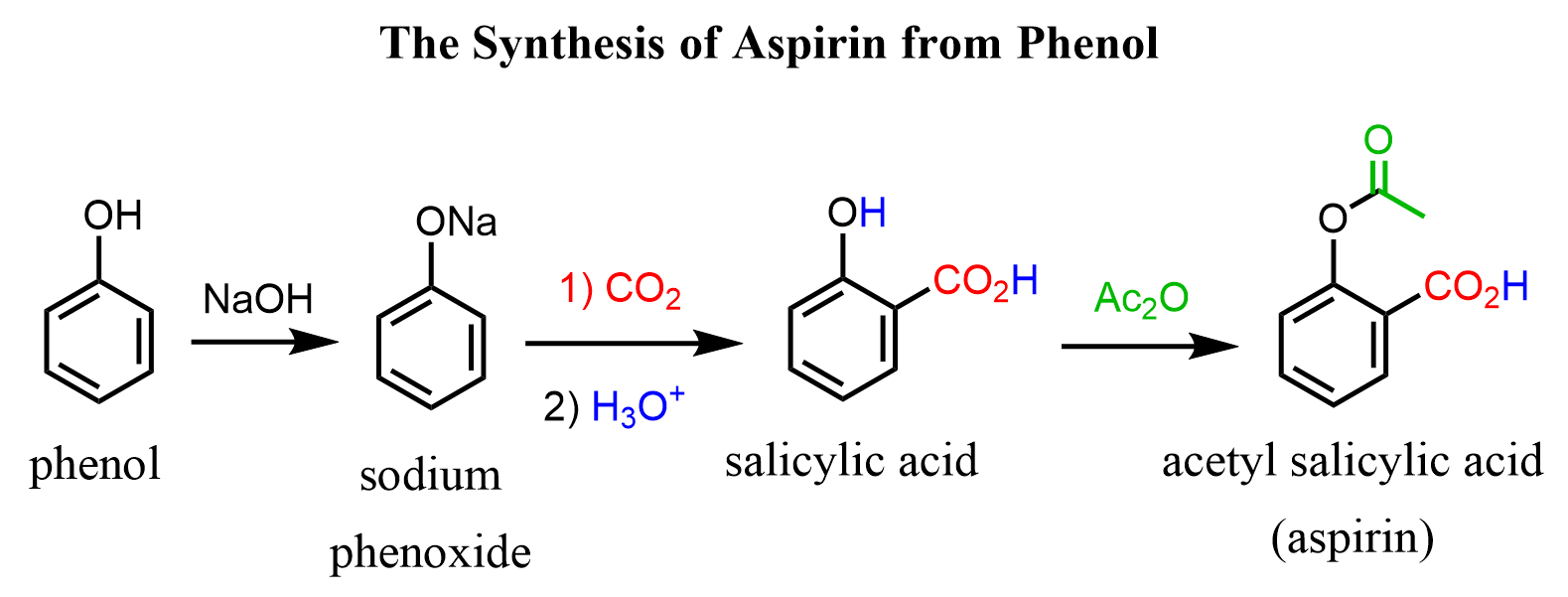
Notice that, unlike monobromination, the carboxylation occurs at the ortho position, which is due to the coordination of the carbon dioxide with the sodium:
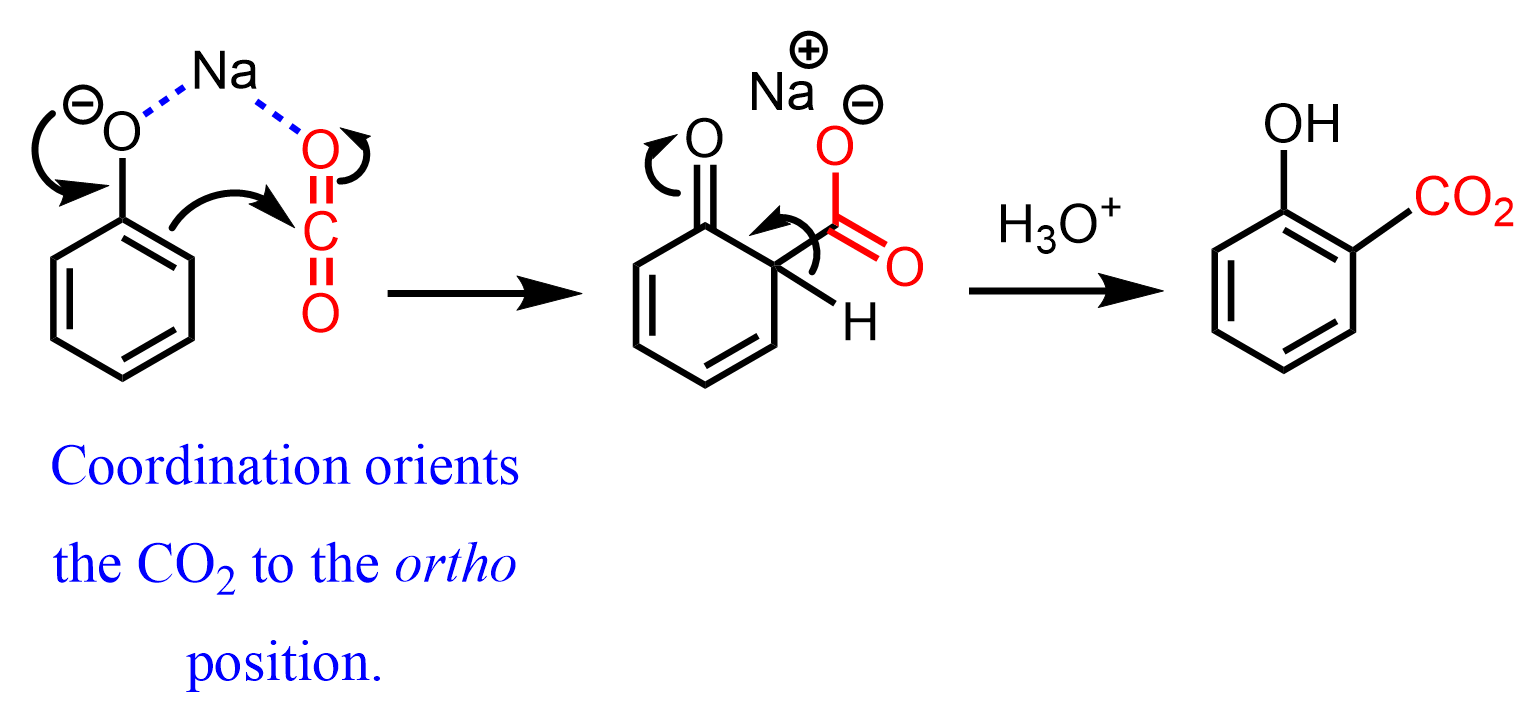
The carboxylation of phenol via converting it first to the phenolate ion is also known as the Kolbe or Kolbe-Schmitt reaction.
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds
