Choosing between SN1, SN2, E1 and E2
In the previous posts, we discussed choosing between SN1 and SN2, as well as SN1, SN2, E1, and E2 mechanisms. The main focus here was on the substrate and the strength of the nucleophile. As a short summary, strong bases/good nucleophiles perform E2 or SN2, while weak bases/nucleophiles can only react by SN1 and E1 mechanisms:

This, as you know, is more complicated, and there are separate posts devoted to this subject.
The Solvent in Substitution and Elimination Reactions
There are so many factors to consider when choosing between SN1, SN2, E1, and E2 that the solvent is often overlooked. However, you may get asked about the effect of solvent on the nucleophilicity and basicity, and that is what today’s post is about.
The solvent is what we use to carry out the reaction, so the main requirement for it is to dissolve the reactants. And because the reactants in nucleophilic substitution and elimination reactions are polar molecules, the solvent needs to be polar as well. The dipole-dipole interaction of these polar molecules with the reactants is what helps to solvate them.
Polar Protic and Polar Aprotic Solvents
There are two types of polar solvents: polar protic and polar aprotic.
Polar protic solvents are capable of making hydrogen bonding, i.e., they contain a hydrogen connected to an electronegative atom and thus can make intermolecular hydrogen bonding in addition to the dipole-dipole interactions. The most common polar protic solvents are water and alcohols.
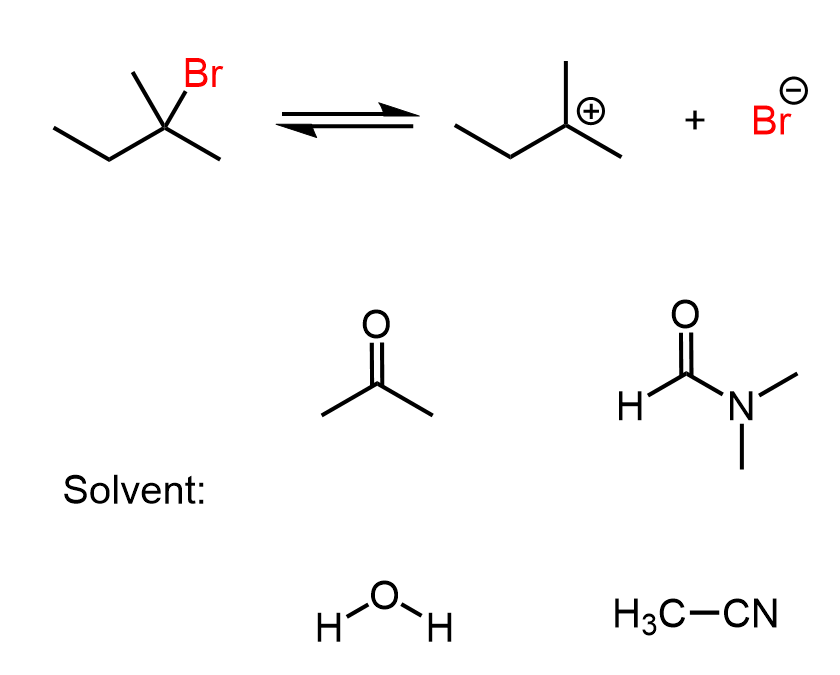
For example, when NaCl is dissolved in water, the sodium ion is solvated, through a dipole-dipole interaction, by the oxygen, and the Cl– ions are solvated by hydrogen bonding with water molecules:

Polar aprotic solvents, on the other hand, are those without a hydrogen connected to an electronegative atom, and the key difference compared to polar protic solvents is the lack of intermolecular hydrogen bonding.
For example, when the salt is added to a solution of DMSO, only the sodium ions are solvated, and the Cl– stays now as a naked ion:

To compare the effect of polar protic and aprotic solvents, we can say that the protic solvent puts the nucleophile in a cage, thus making it weaker, while the polar aprotic solvent solvates the cation, leaving the nucleophile free. As a result, in the polar aprotic solvent, it becomes a stronger nucleophile since the counterion does not reduce its reactivity.
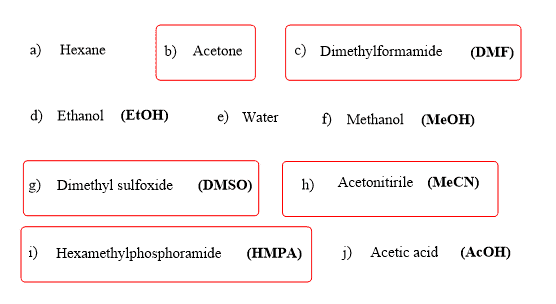
There is a large group of common polar aprotic solvents, such as acetone, acetonitrile, DMF, DMSO, HMPA, shown below:

The Effect of Solvent on Nucleophilicity and Basicity
Now, the important question: How does this behavior of polar protic and polar aprotic solvents affect the nucleophilicity and basicity?
Let’s remember that very often nucleophilicity parallels basicity, meaning the stronger base is also the stronger nucleophile. Therefore, if we leave the solvent alone for a moment, we can say that the basicity and nucleophilicity increase as we go up the periodic table:

Check this article about the factors affecting the pKa to refresh the concept of stronger acids and bases.
However, it is not always that basicity goes with nucleophilicity.
For example, the ethoxide ion is an excellent nucleophile and a strong base. However, as we make the molecule bulkier (sterically hindered), it becomes a weak nucleophile but still a strong base, even though the negative charge is still on the same atom:
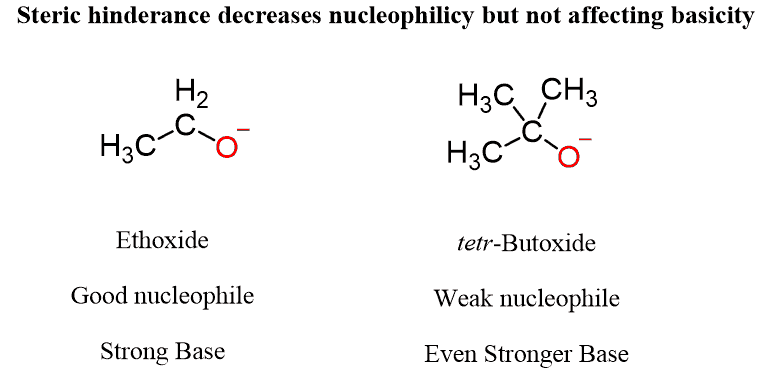
What is going on here?
To understand this pattern, remember that the base needs to only access a small proton, while the nucleophile needs to access a carbon atom, which is often hindered by neighboring hydrogens and neighboring carbon atoms. This is why larger molecules lose their nucleophilicity while retaining the base strength:

The effect of a polar protic solvent is similar to this since it forms hydrogen bonds with the nucleophile, thus putting it in a cage and making it sterically hindered. As we have seen above, this enlarged species is now a weaker nucleophile, yet their basicity is largely intact:
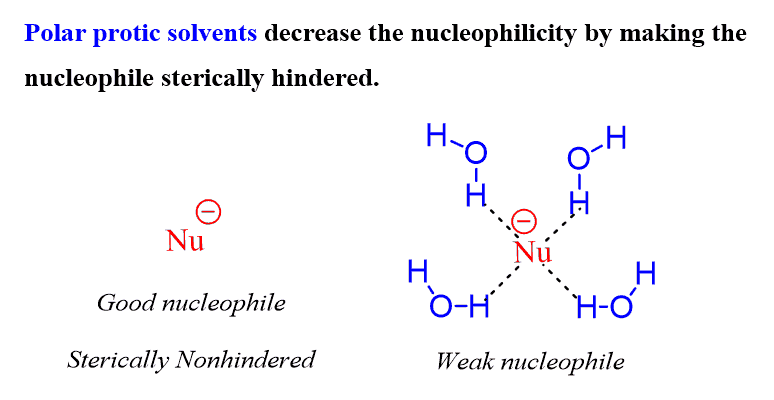
If we compare this with basicity, which is not affected, the result of a polar protic solvent is that the nucleophilicity is reversed, and it is now opposite to basicity.
A polar aprotic solvent, on the other hand, does not interact with the nucleophile since there is no hydrogen bonding. Therefore, the basicity and nucleophilicity are not affected, and they still go parallel:
The chart below is a summary of the solvent effect on nucleophilicity and basicity:

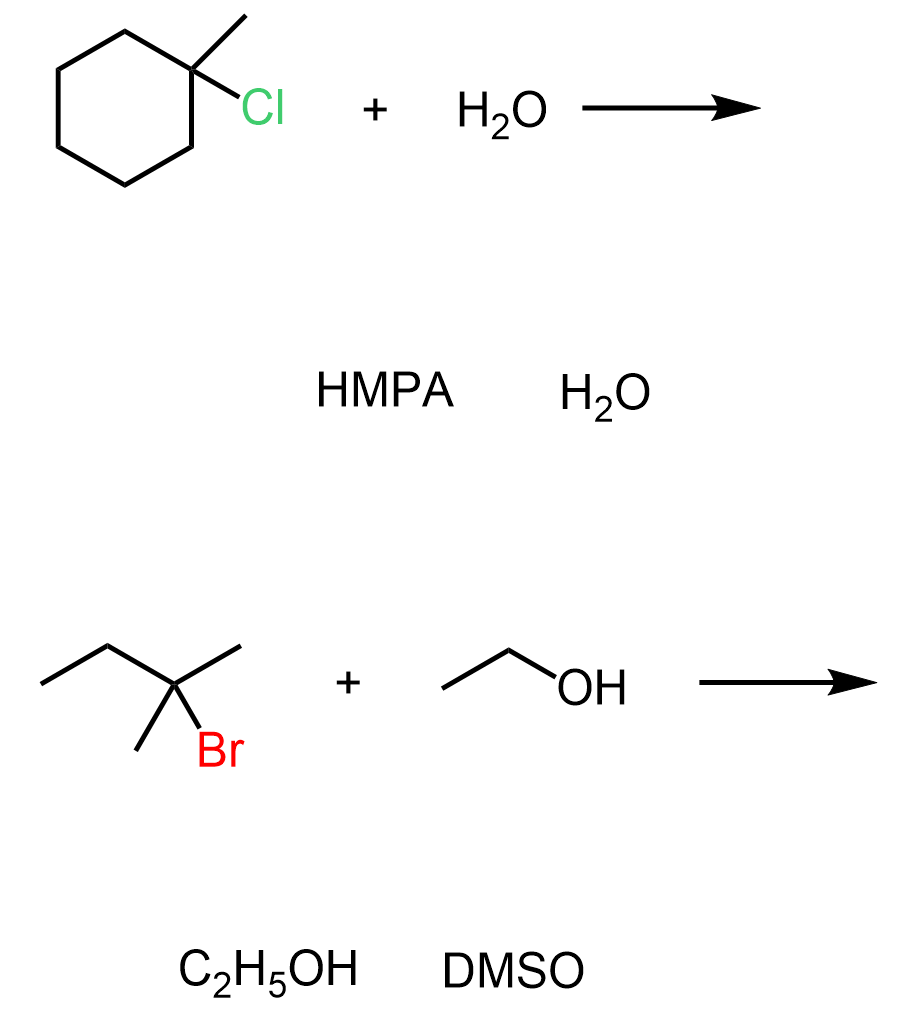
The Effect of the Solvent in SN1 and SN2 Substitution Reactions
If you need to choose between SN1 and SN2, then remember that polar aprotic solvents favor SN2, while polar protic solvents favor the SN1 mechanism since the nucleophilicity in this case is decreased. Don’t forget that strong nucleophiles perform SN2- they do not need to wait for the carbocation to be formed once the leaving group is gone:

I have also addressed the effect of solvent in substitution reactions in this detailed post about the SN2 mechanism.
The role of solvent in SN1, SN2, E1, E2 competition
To keep this as simple as it can be, first remember that the solvent is not the first factor you consider when choosing between SN1, SN2, E1, and E2.
The first step is to determine if you have a strong base/nucleophile or a weak base/nucleophile.
If strong, you are between SN2 or E2.
If weak, it is either SN1 or E1.
For any of these pairs, remember that protic solvents will favor elimination over substitution since caging the nucleophile by hydrogen bonding decreases the nucleophilicity since it makes the nucleophile bulkier and more difficult to reach the carbon with the leaving group.
This effect is not significant on the basicity since even protonated and caged species can access the b-proton and thus serve as a base.



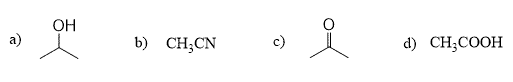

Education should be free GOOD STUFF but I don’t get any $$ to spare for this
Thanks for the comment. As you can see, there is plenty of free content not only to read but also to practice. Unfortunately, everything cannot be free. We cannot work for free. I spent thousands of hours creating this, and it is not free even for me, the author, as I need to pay for hosting, maintenance, and supporting staff. Most platforms, such as Khan Academy, that are free have sponsors and that’s how they sustain the production. We don’t and only rely on the support of our students-no ads either. Please kindly share here other resources with as much content that are free so that everyone can benefit.
This was very helpful. Thank you!
Glad to hear that, Michelle.
Awesome. Thank so much !
Thank you, Yasmine.
I really apprciate your effort on these contents, thank you very much!
Thanks, Jimin.