One of the most common questions in organic chemistry is whether a reaction follows the SN2 or E2 mechanism. So, how do you know which one it is when you’re given an alkyl halide or similar substrate?
This is part of the bigger decision tree for SN1, SN2, E1, or E2 reactions, which we’ve covered in a separate post (make sure to check that out).
For now, just remember this:
You only consider SN2 or E2 when a strong nucleophile/base is involved.
If the reaction uses a weak base or a poor nucleophile, then you’re looking at either SN1 or an E1 reaction.

So, in today’s discussion about SN2 and E2 reactions, we are on the right side of this branching. Once again, if you see a weak base or a nucleophile such as water or an alcohol given, you are most likely looking at the left branch of deciding between SN1 and E1 reactions.
The Role of Base and Nucleophile in Deciding Between SN2 and E2 Reactions
You have determined that a strong nucleophile/base is used; therefore, it must be an SN2 or an E2 reaction. The next question is whether this base/nucleophile tells you anything in choosing between SN2 and E2.
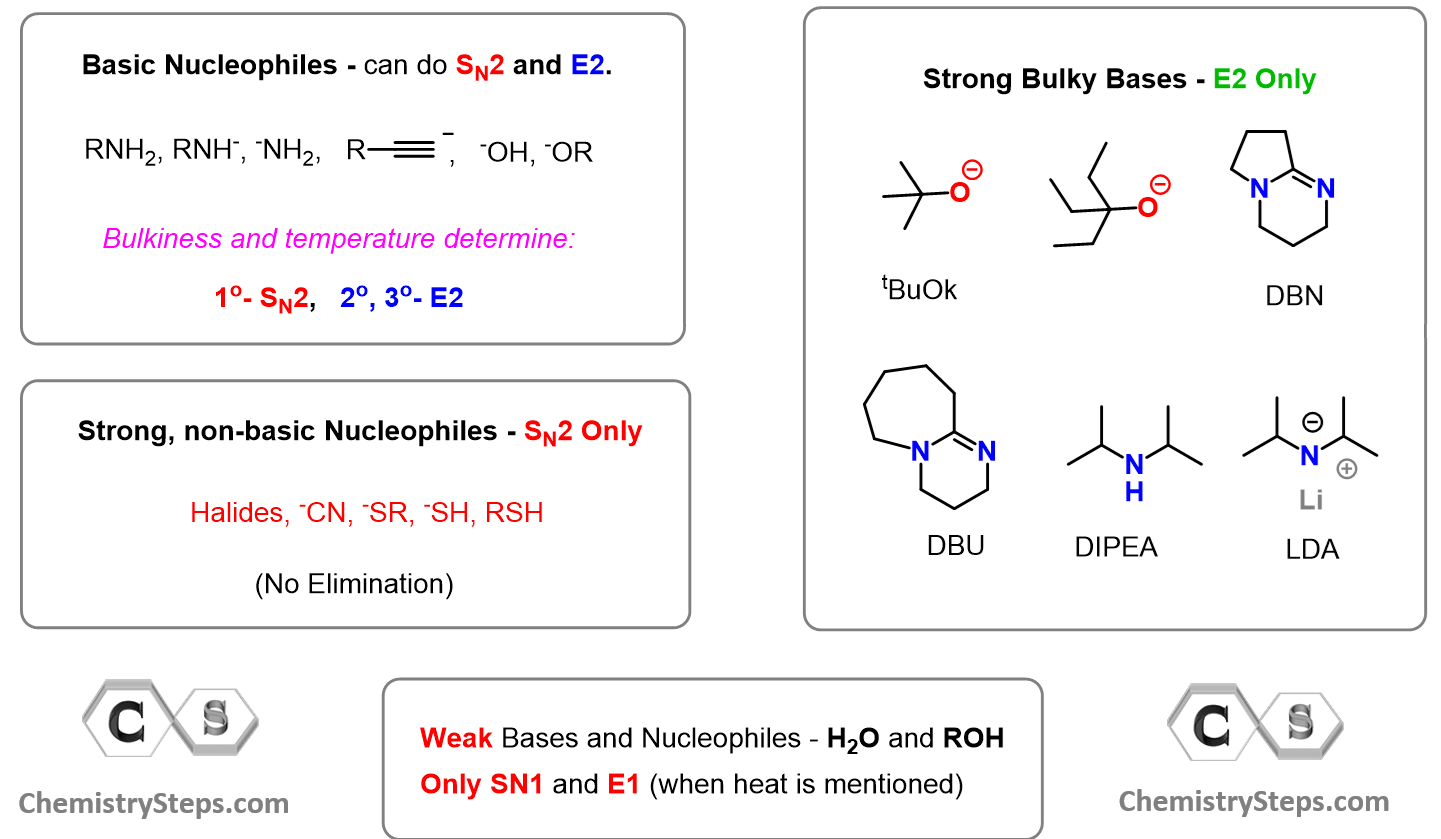
The answer is yes, the structure of the reagent can be very helpful in choosing the correct mechanism. For this, we classify them as either basic or non-basic nucleophiles. Basic nucleophiles are those that, in addition to being good nucleophiles, are also strong bases. Non-basic nucleophiles are the good guys, as they only do SN2 substitution. These are mainly halides, cyanide, sulfide ions, as well as the thiols themselves.
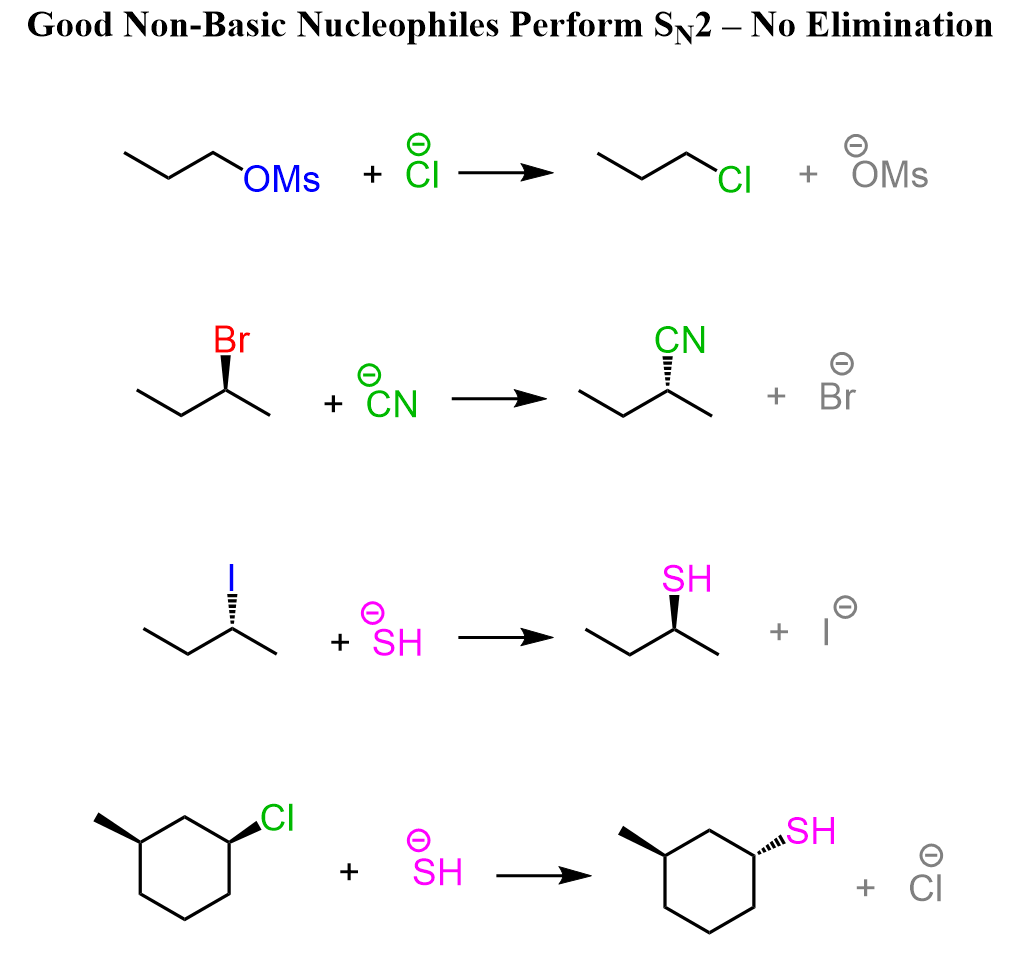
Charged species of oxygen and nitrogen – the conjugate bases of alcohols, water, and amines, and any hydrocarbon are the basic good nucleophiles. These species act as nucleophiles and do SN2 with primary alkyl halides, while the E2 mechanism predominates with secondary and tertiary alkyl halides.
Note: We are calling them basic nucleophiles because they are often nucleophiles, but when reacted with secondary or tertiary alkyl halides, it is more accurate to call them bases.
For example, see how hydroxide and acetylide ions give SN2 and E2 products with primary and secondary alkyl halides, respectively:
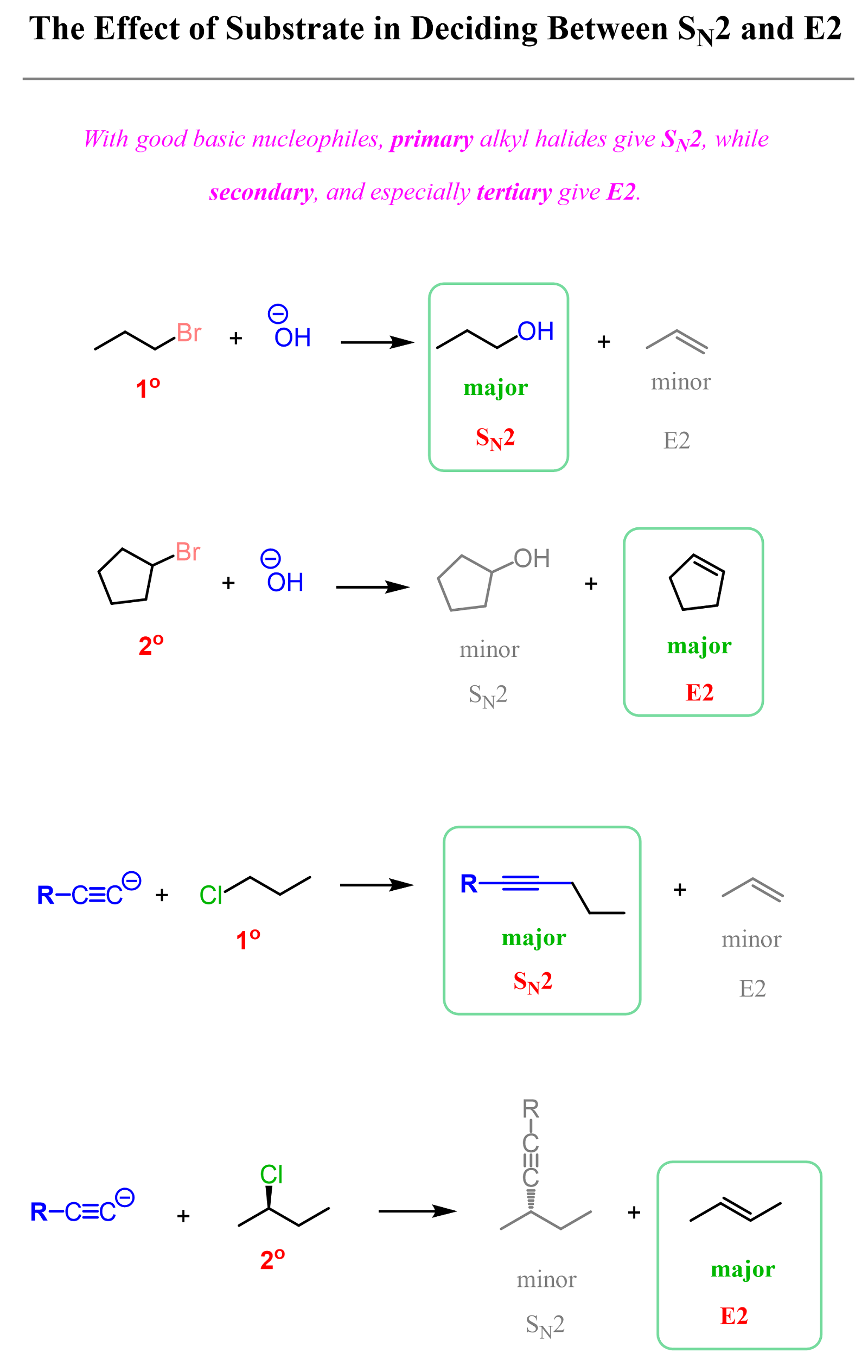
So, primary alkyl halides favor SN2 substitution unless, as mentioned above, a bulky strong base is used. Anything that is not bulky favors SN2 with primary alkyl halides. On the other hand, if a secondary, and of course, a tertiary alkyl halide is used, the E2 elimination predominates, though the SN2 product may also be formed in smaller quantities.
The Role of Steric Hindrance in SN2 and E2 Reactions
If the reagent is a strong, bulky base such as t-BuOK, DBU, or DBN, then regardless if the substrate is primary, secondary, or tertiary, the E2 elimination will predominate exclusively because these are too sterically hindered. Remember, we learned SN2 is difficult for sterically hindered substrates because the nucleophile can’t access the electrophilic carbon bearing the leaving group. Now, molecules do not classify themselves as nucleophiles and substrates, so it does not matter which one of these is sterically hindered – the outcome is the same, E2 elimination.
For example, 1-bromopropane is a primary alkyl halide, and it gives an SN2 product when reacted with an ethoxide ion (non-hindered strong base/nucleophile), whereas the reaction with tBuOK, a sterically hindered strong base, gives E2 elimination.
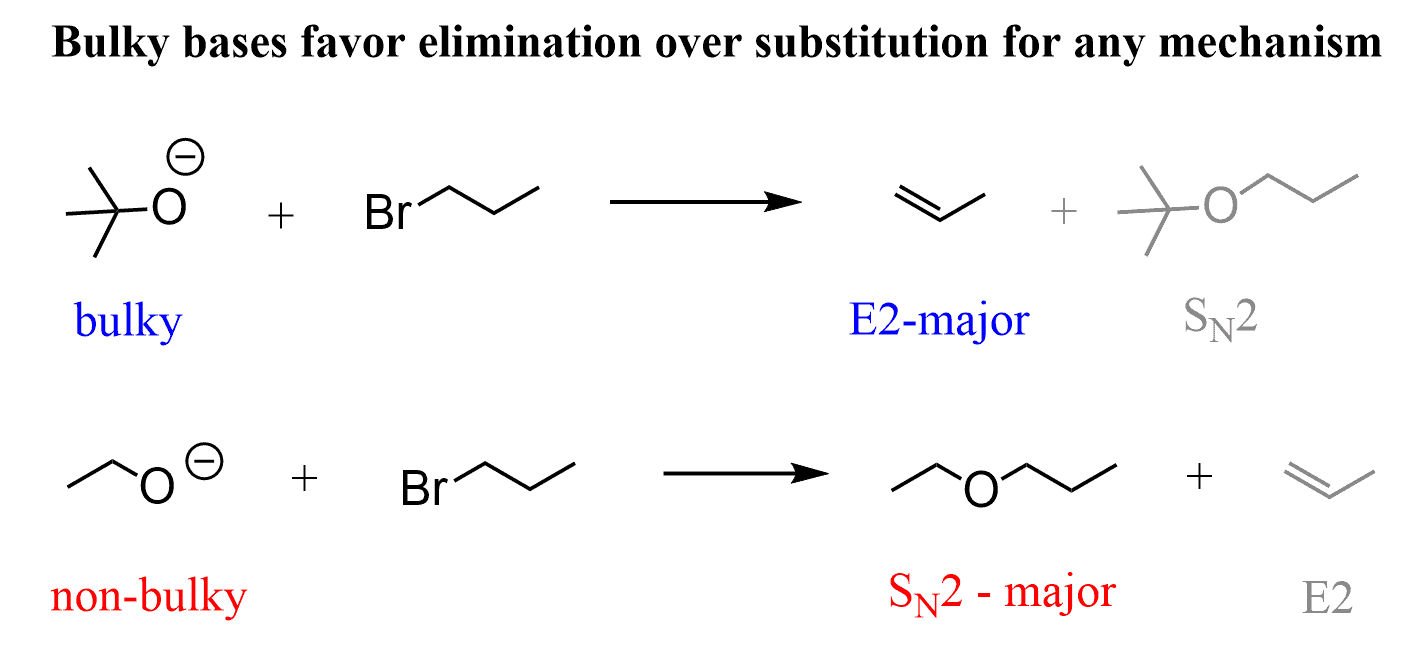
To understand this pattern, remember that the base needs to only access a small proton, while the nucleophile needs to access a carbon atom, which is often hindered by neighboring hydrogens and neighboring carbon atoms. This is why larger molecules lose their nucleophilicity while retaining the base strength:

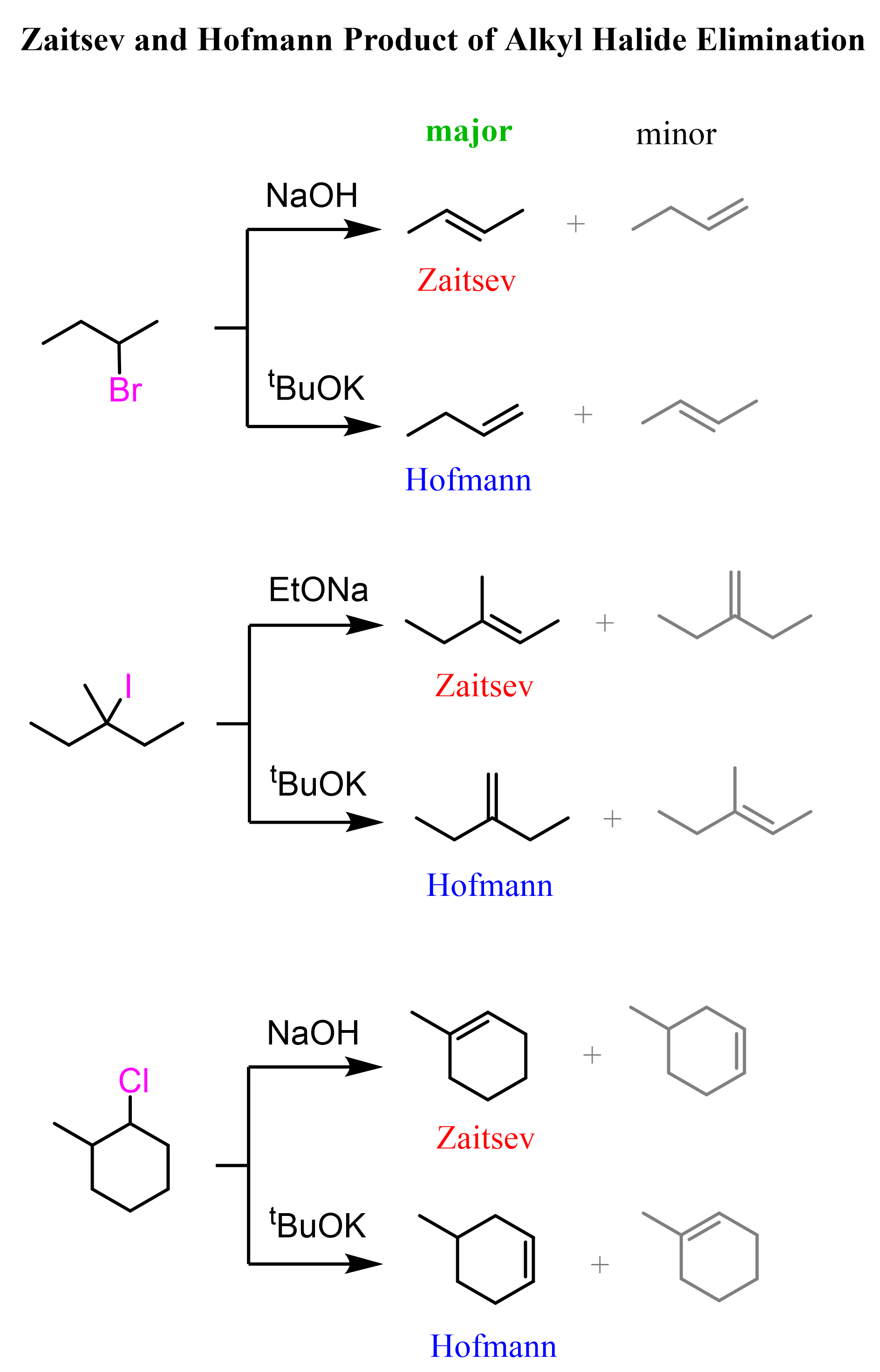
On a side note, here is also a reminder that the regiochemical outcome of E2 reactions depends on whether a bulky on ninhindereder base is used:
Relatively small bases (sterically non-hindered or non-bulky), such as hydroxide ions, favor the formation of more substituted alkenes. This is known as Zaitsev’s rule. On the contrary, bulky bases (sterically hindered) such as potassium tert-butoxide (tBuOK) favor the formation of less substituted alkenes – Hofmann elimination:
Summary on Deciding Between SN2 and E2 Reactions
SN2 and E2 are bimolecular reactions that occur when strong bases or good nucleophiles are used. These reactions happen in a single concerted step, meaning no intermediates like carbocations are involved.
The outcome depends on both the substrate (alkyl halide) and the reagent (nucleophile/base)
- SN2 is favored with primary substrates and non-bulky good nucleophiles
- E2 is favored with strong, bulky bases
- Any combination of strong-bulky, whether it is the substrate or the reagent that is bulky, gives E2 elimination
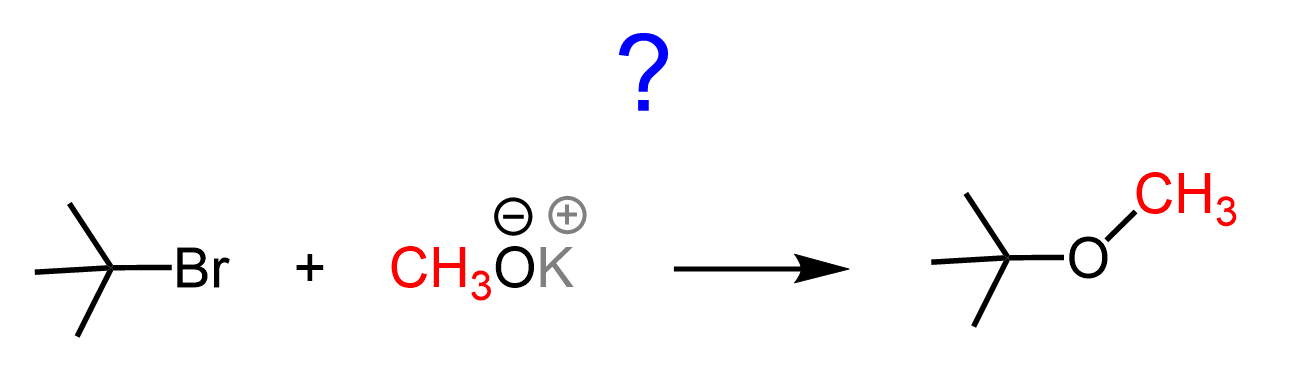
OK, we are almost done, and I hate to do this, but you need to remember there is always an exception to “always,” “any,” “never,” etc. If we react t-BuOK with a methyl halide, there simply cannot be an E2 product, regardless of how bulky it is.
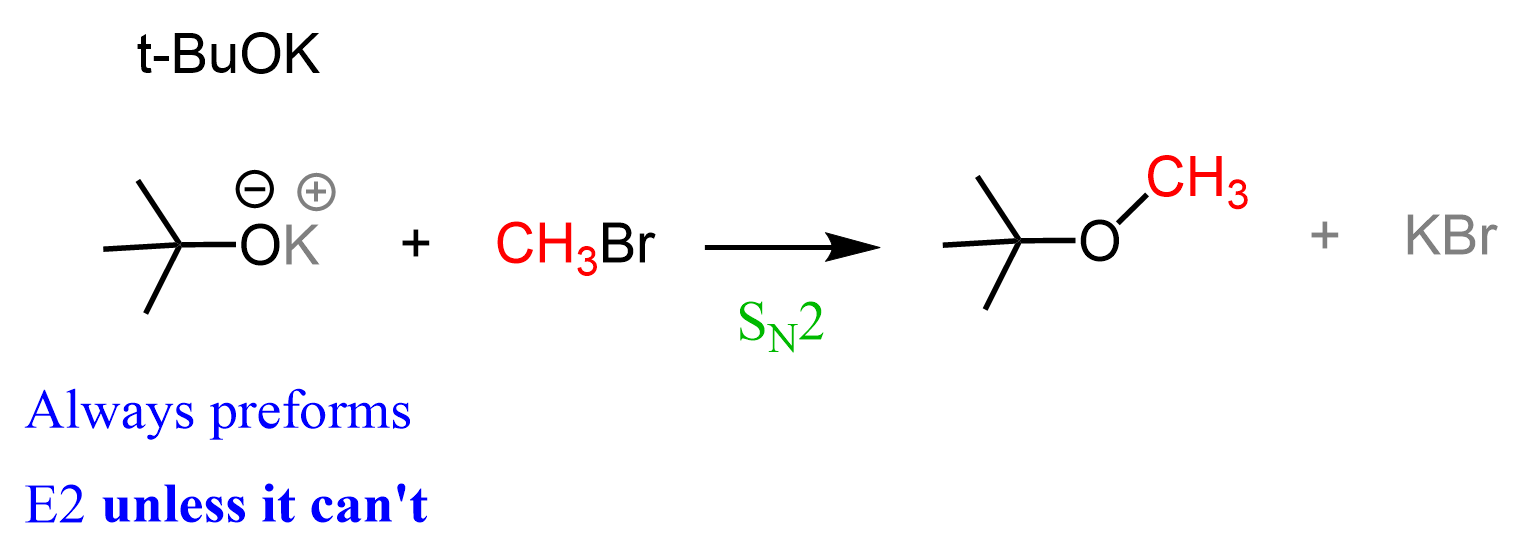
So, don’t just rely on memorizing the rules and patterns – always give a second thought, especially if you feel like the problem cannot be so easy, knowing your professor its point value.
Do you think this reaction would work?