We learned in general chemistry that the oxidation state is determined based on the number of bonds connecting elements with different electronegativities. Very often though, we didn’t need/bother to look at the number of bonds, but simply used a formula to calculate the oxidation states. For this, we used the value for the element with standard electronegativity and did the calculation based on that. For example, to determine the oxidation number of carbon in CO2, we first look up the standard oxidation state of oxygen (-2), assign x for the carbon, and solve for it keeping in mind that the sum of the oxidation states in a neutral molecule is 0:
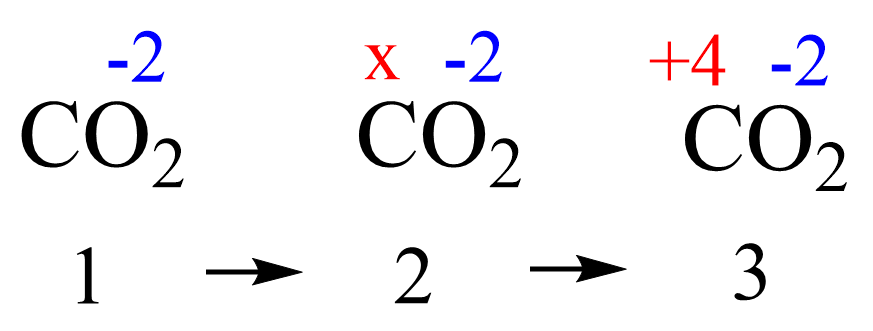
x + 2 (-2) = 0
x = +4
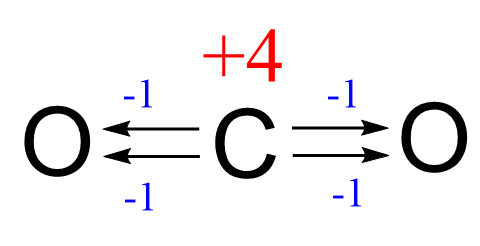
Now, we can also see this by drawing out the structure of CO2. Remember that each bond of a more electronegative atom increases the oxidation state by one, and because there are four bonds connecting to the oxygens, the oxidation state of the carbon is +4.
Another example is determining the oxidation states in CH2O. If we didn’t know the structure of this molecule, we’d assign +1 for the hydrogen, -2 for the oxygen, and x for the carbon:
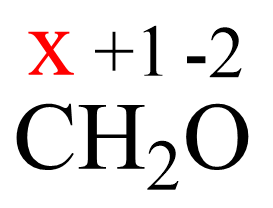
x + 2 (+1) + (-2)= 0
x = 0
Recall the standard oxidation states:
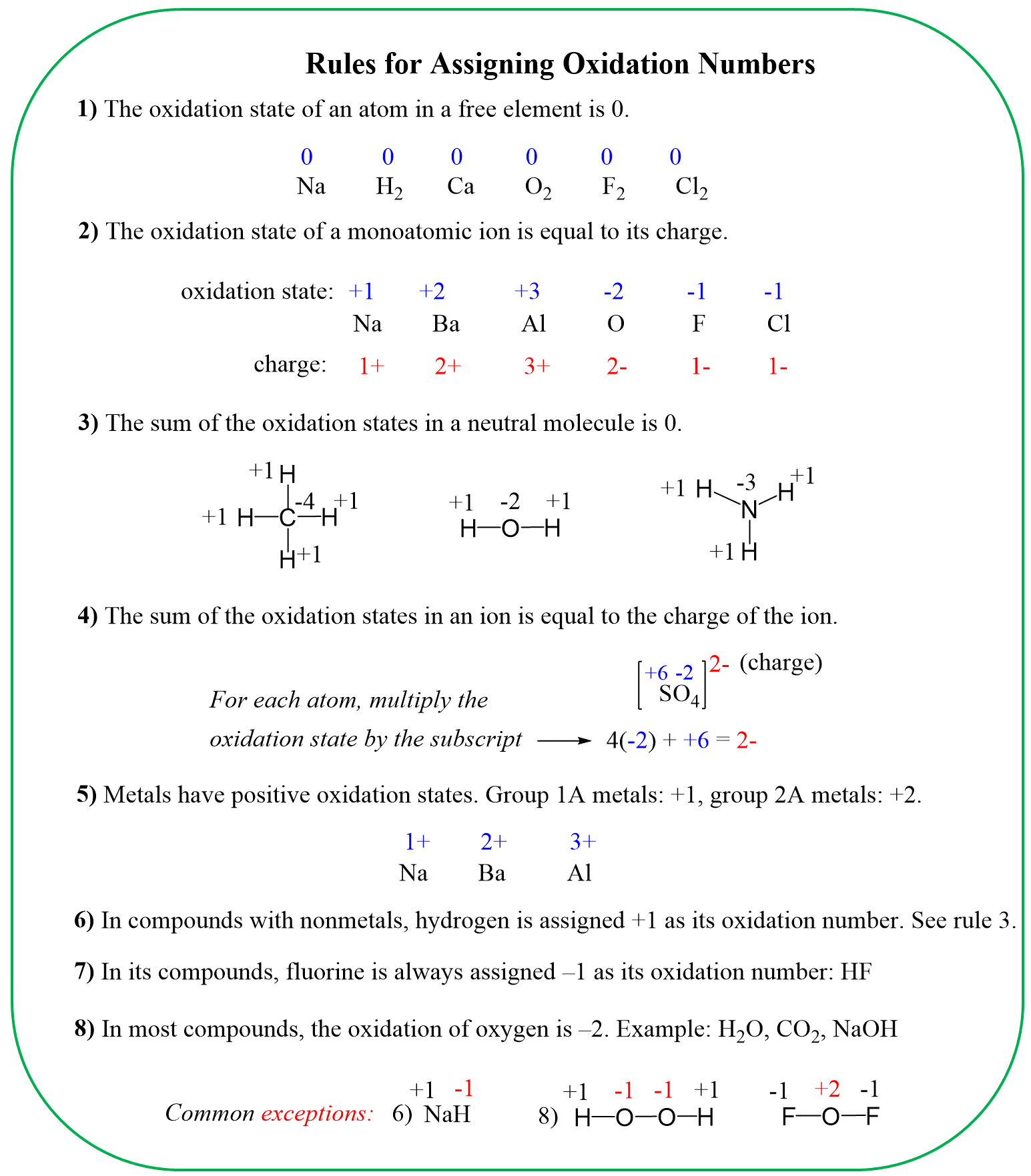
Let’s now look at the structure of this molecule (formaldehyde):
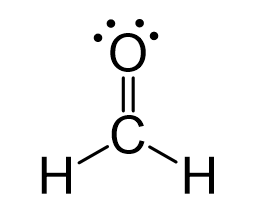
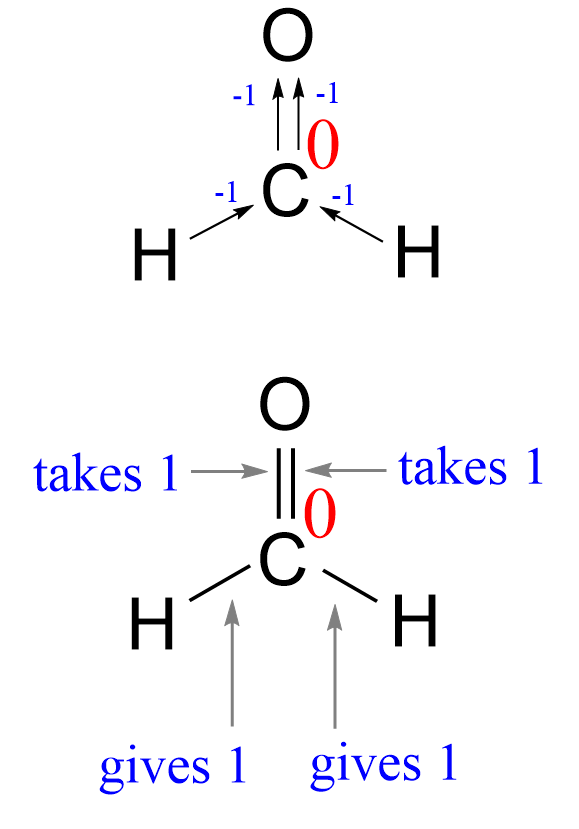
There is an oxygen connected to the carbon, and because it is double-bonded, we count it as two oxygens. This gives +2 to the oxidation state of the carbon. However, there are also two hydrogen atoms, which are less electronegative than carbon (2.1 vs 2.5). These, in turn, give -2 to the carbon, and therefore the overall oxidation state sets to zero:
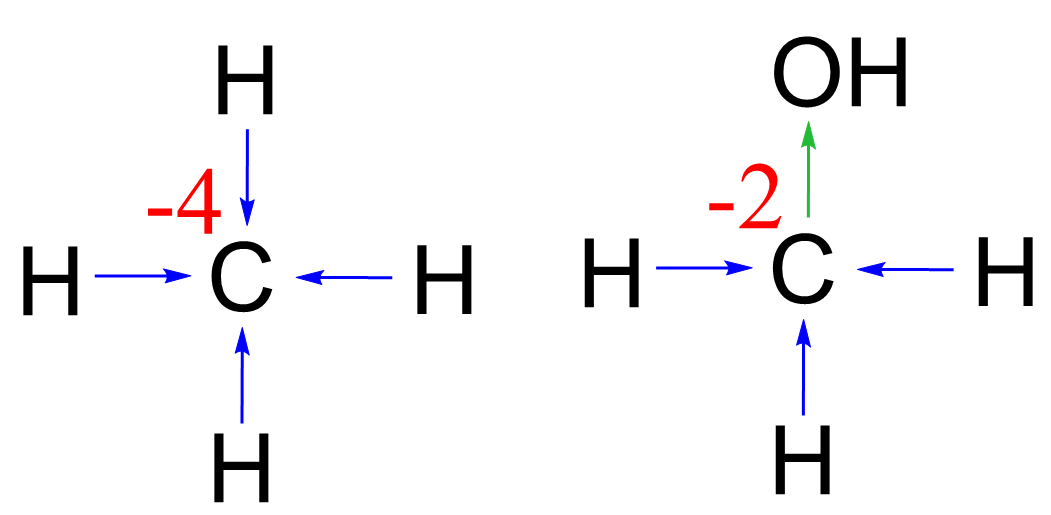
Two quick observations we can make here: oxygen increases the oxidation state of carbon, whereas hydrogen decreases it. So, for a simple molecule like CH4, we can clearly see that the oxidation state of the carbon must be -4. In methanol, for example, CH3OH, it would be -2 because instead of a hydrogen, there is an oxygen connected to the carbon, and it takes one ‘negative charge’ from the three given by the three hydrogens:
Recall that oxidation numbers are formalities and the atoms are not actually charged unless it is an ionic compound.
We also know that carbon does not affect the oxidation state of another carbon it is bonded to. This is true for any element – the same elements do not affect each other’s oxidation states. For example, in ethane, each carbon is connected to three hydrogens and one carbon, thus the oxidation state is -3:
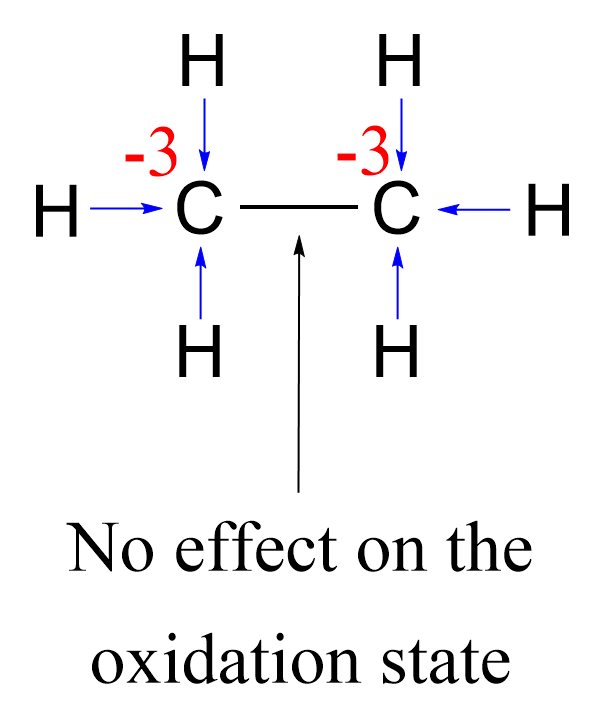
Comparing this with the oxidation state of the carbon in methanol, we anticipate that in primary alcohols with longer carbon chains, the oxidation state of the carbon connected to the oxygen will increase by one because instead of a hydrogen, there will be another carbon.
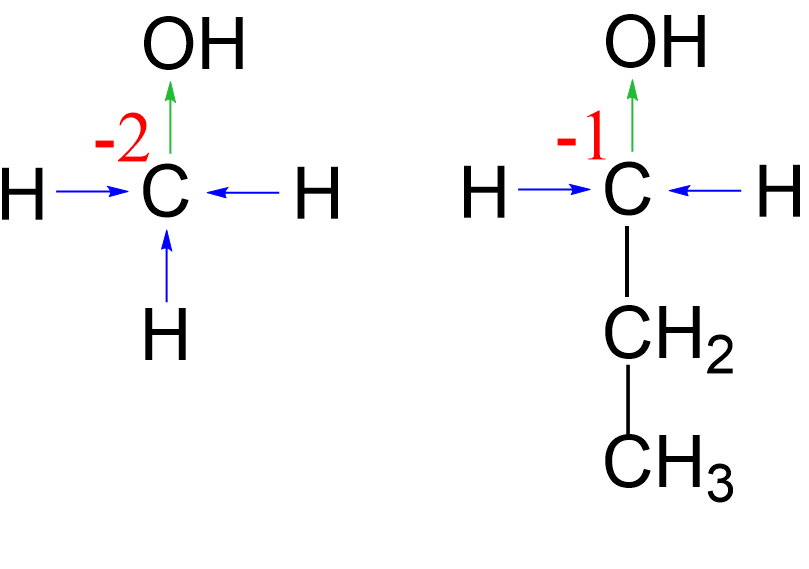
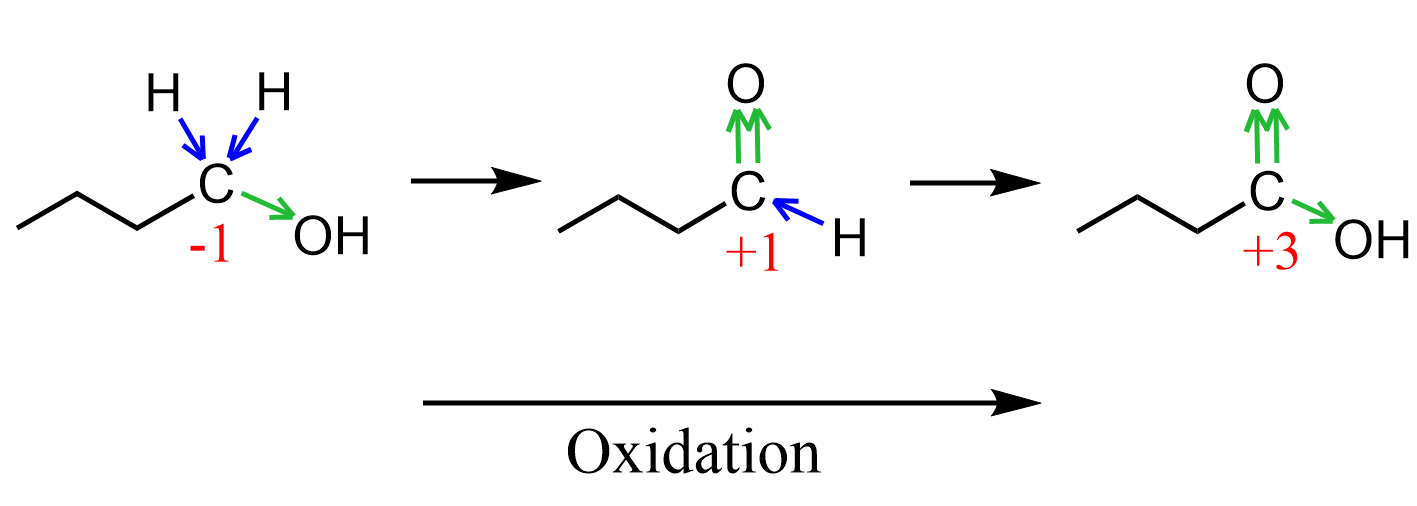
Summarizing what we have seen so far, we can see that replacing a hydrogen with an oxygen increases the oxidation state of the carbon, and this explains why the conversion of alcohols to carbonyl compounds is classified as oxidation, and the revere process as reduction:
The conversion of alkanes to alkyl halides is also an example of oxidation because halogens are more electronegative than carbon. So, we can draw scheme involving an alkane to carboxylic acid oxidation conversion:

Oxidation Reactions in Organic Chemistry
Let’s recall some examples of oxidation reactions. As the name suggests, hydroboration-oxidation is an oxidation reaction of alkenes to alcohols, and so is the acid-catalyzed hydration:

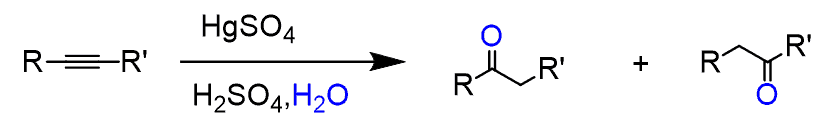
The same is the hydroboration oxidation of alkynes, which converts them to aldehydes or ketones:
The subject of oxidation becomes relevant in the chapter on alcohols and carbonyl compounds such as aldehydes, ketones, carboxylic acids, and their derivatives. Below is a short summary of the oxidation of alcohols using a variety of reagents, and you can find more details about it here:

Reduction Reactions in Organic Chemistry
The reverse process of oxidizing molecules is their reduction. Most often, in organic chemistry, these are going to be the reduction reactions of carbonyl compounds to the corresponding alcohols. For example, common reducing agents such as LiAlH₄ and NaBH₄ can be used to reduce aldehydes and ketones to primary and secondary alcohols, respectively.

There are, of course, other reduction reactions, which can yield different outcomes depending on the structure of the substrate and the reducing agent. For example, NaBH4 does not normally reduce esters; however, we can reduce them to either alcohols or aldehydes depending on the reducing agent:
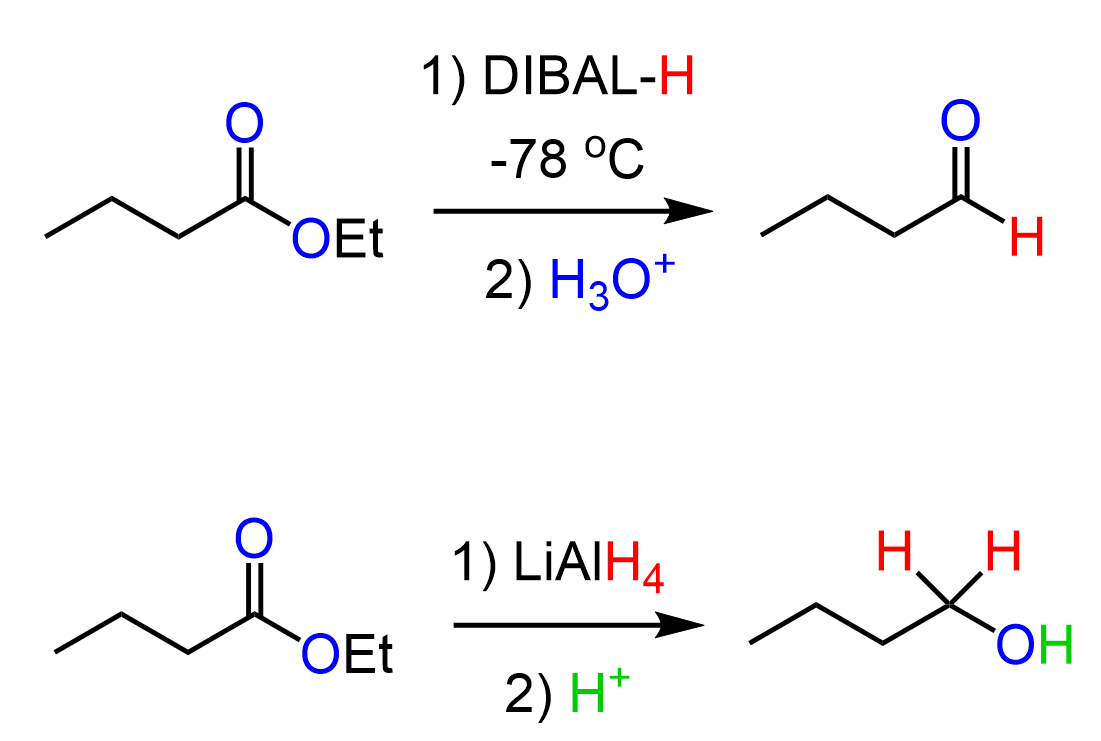
The links to these conversions will also be listed below this discussion.
Summarizing Oxidation States in Organic Chemistry
To summarize what we have discussed, remember that oxygen increases the oxidation state of carbon, while hydrogen decreases it. Carbons bonded to other carbons do not affect each other’s oxidation states.
Replacing a hydrogen with an oxygen atom (as in converting alcohols to carbonyls) is classified as oxidation, while the reverse is reduction. The same principle applies to many transformations, such as oxidizing alkanes to halides or converting alkenes/alkynes through hydroboration–oxidation.
Reduction is the reverse process, which most often is the conversion of carbonyl compounds back to alcohols using reducing agents like LiAlH₄ and NaBH₄, though other pathways are also possible depending on the substrate and reagent.
Key takeaway:
-
More bonds to oxygen (or other electronegative atoms like halogens) → higher oxidation state → oxidation.
-
More bonds to hydrogen → lower oxidation state → reduction.
This framework provides a simple way to recognize and classify oxidation and reduction reactions in organic chemistry.
Check Also
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Reduction of Aldehydes and Ketones
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carboxylic Acids and Their Derivatives
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- DIBAL Reducing Agent
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols
