Let’s talk about the Wittig reaction, which is used for converting aldehydes and ketones to alkenes:
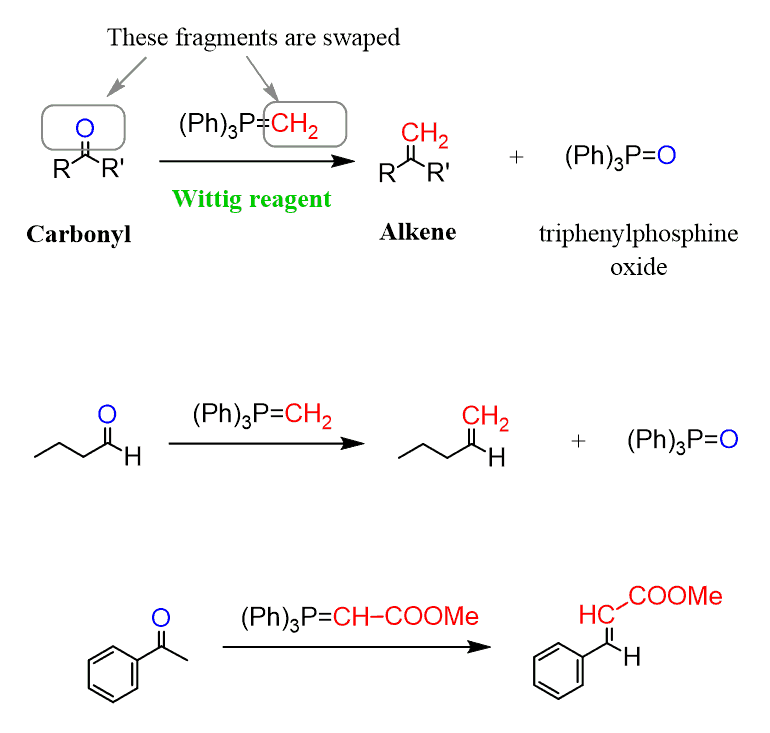
So, this is, in a way, the reverse reaction of the alkene ozonolysis, where the C=C bonds were cleaved to form carbonyl groups:

The reaction is not restricted to only adding a methylene group (CH2) and, in theory, a carbon chain with any functional groups can be installed on the C=O carbon atom. It is especially common to have a Wittig reagent with an electron-withdrawing group, such as esters, which stabilize the negative charge of the carbon connected to the phosphorus and also influence the stereochemistry of the reaction.
The Wittig reaction is a very important tool in organic chemistry, which is used not only in the labs but also in industry for the synthesis of β-carotene and vitamin A derivatives:

Because of its tremendous importance, the Wittig reaction earned Georg Wittig (1897–1987) the 1979 Nobel prize in Chemistry.
Wittig Reaction Mechanism
Let’s now discuss the mechanism of the Wittig reaction. It is a nucleophilic addition-elimination reaction and, in that sense, is still somewhat like the other reactions of aldehydes and ketones, such as the ones with cyanides, alcohols, or amines.
However, the mechanism is a little different and involves a cyclic intermediate called oxaphosphetane.
The nucleophilic center in the Wittig reagent is the carbon connected to the phosphorus.
In general, the Wittig reagent can be shown by two resonance structures; one with a double bond between the phosphorus and the carbon, and the second, where these atoms have opposite formal charges – this is the ylid:

Ylids are a class of compounds where covalently bound atoms, each with an octet, have opposite charges:
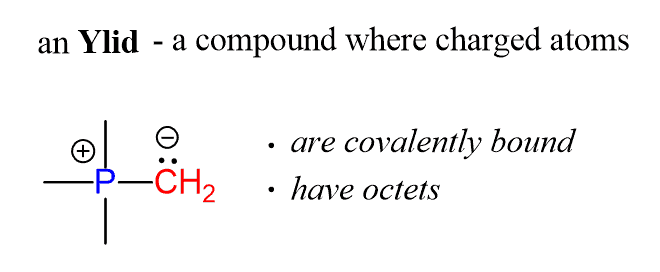
Despite bearing formal charges, the ylid is still an important resonance contributor since the atoms have octets.
The ylid form of the Wittig reagent also helps to better visualize the reaction mechanism. It is believed to be a concerted [2+2] cycloaddition process. The negatively charged carbon atom of the ylide attacks the carbonyl carbon, moving the π bond electrons toward the oxygen which, in turn, attacks the positively charged P atom:

The [2+2] notation refers to the number of atoms participating in the reaction. In this, it is the two atoms of the carbonyl group and the P and O from the Wittig reagent. The Diels-Alder reaction, for example, is a [4+2] cycloaddition reaction – four atoms from the diene and two from the dienophile.
The [2+2] addition forms a four-membered ring called oxaphosphetane made of new carbon-carbon and oxygen-phosphorus σ bonds.
This intermediate is not very stable and undergoes an intramolecular elimination to give the alkene and triphenylphosphine oxide as a by-product.
Depending on your instructor, it might be acceptable to show the Wittig reaction as a stepwise mechanism, which helps to show how the bonds are made. In this case, we show the nucleophilic addition first and then the P-O bond formation using the lone pairs on the negatively charged oxygen:

Wittig Reagent Preparation
The Wittig reagent is prepared in tow steps using triphenyl triphenylphosphine (Ph3P) and an alkyl halide. First, the Ph3P acts as a nucleophile, replacing the halide in an SN2 reaction to give a phosphonium salt:
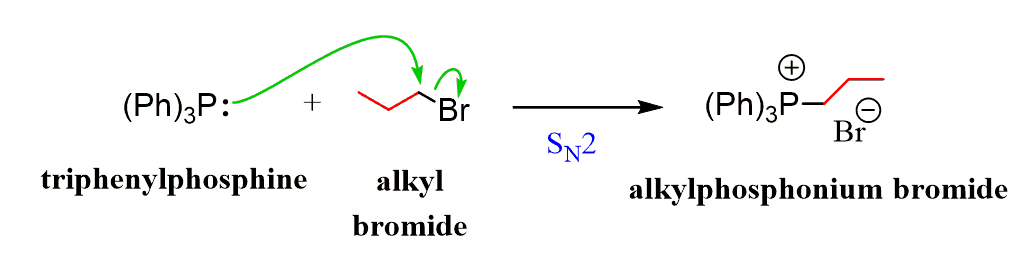
In the phosphonium salt, the carbon is connected to a positively charged phosphorus capable of accepting another pair of electrons (P can exceed the octet). This makes it possible to remove the hydrogen adjacent to the phosphorus with a strong base such as an organolithium reagent, forming the desired ylid:

The fact that the preparation of a Wittig reagent involves an SN2 reaction brings up additional considerations when designing alkene synthesis. This retrosynthetic analysis for the Wittig reaction and some more practice problems are covered in the next post:
Wittig Reaction – Practice Problems
Check Also
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- Grignard Reaction with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

Interesting very.I love chemistry. 🙂
Hello, why don´t you put references, and a tool so user que easily cite this work. Thank you.
Hi,
Most of the content and practice examples are general knowledge rather than a specific advanced topic or a new research discovery. For example, here, I did not go to the papers published on the discovery and basic advancement of the Wittig reaction.
You can use undergraduate-level textbooks such as Smith, Bruice, Klein, Loudon, Brown, etc., and for more advanced reading – Clayden, March, Bruckner, and Anslyn’s Physical Organic Chemistry is what comes to my mind.