The SN1 nucleophilic substitution is a unimolecular – first-order reaction:

It is a stepwise mechanism that starts by breaking the bond of the α carbon and the leaving group, followed by the nucleophilic attack:

As you can see, the nucleophile does not appear in the rate equation which means it has no impact on the rate of the SN1 reaction.
It is interesting, because why wouldn’t it?
It does react with the carbocation and increasing its concentration should also increase the rate of the reaction, shouldn’t it?
Well, remember that there is what’s called a rate-determining step of the reaction. It is the step that controls the overall rate of the reaction, as it is the slowest and the process does not get faster no matter how fast the other steps occur.
To highlight the slow (rate-determining) step of the SN1 mechanism, I will show the reaction of an alkyl halide with water (hydrolysis or solvolysis in a more general sense), as there are two fast steps here:
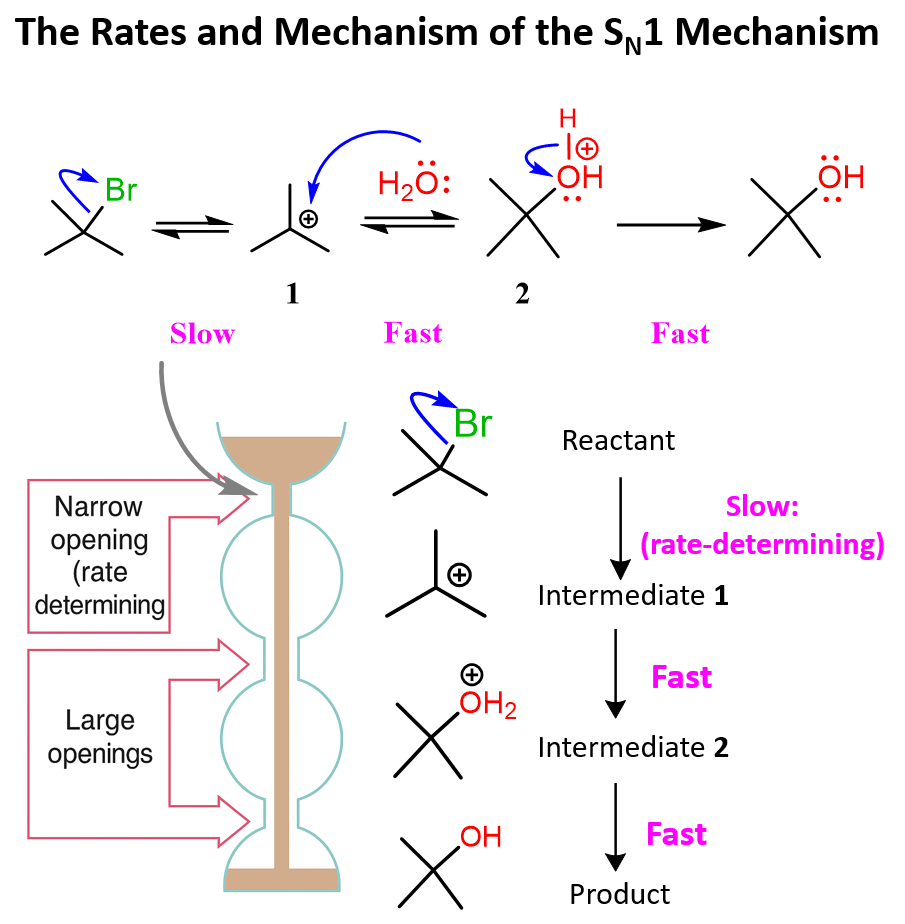
There is one more question to address: why is the loss of the leaving group the rate-determining step?
Simply put, you can look at it his way; a loss of the leaving group means breaking a “healthy” σ bond for no apparent reason:

There is no good reason why the molecule should do it. It only happens in small percentages, facilitated by the polar media (polar solvents) that can ionize and then stabilize the resulting carbocation. One factor that plays in favor of this process is the increase in entropy.
Once a small amount of the carbocation is formed, it then quickly reacts with the nucleophile because of the positive charge, making it very reactive:
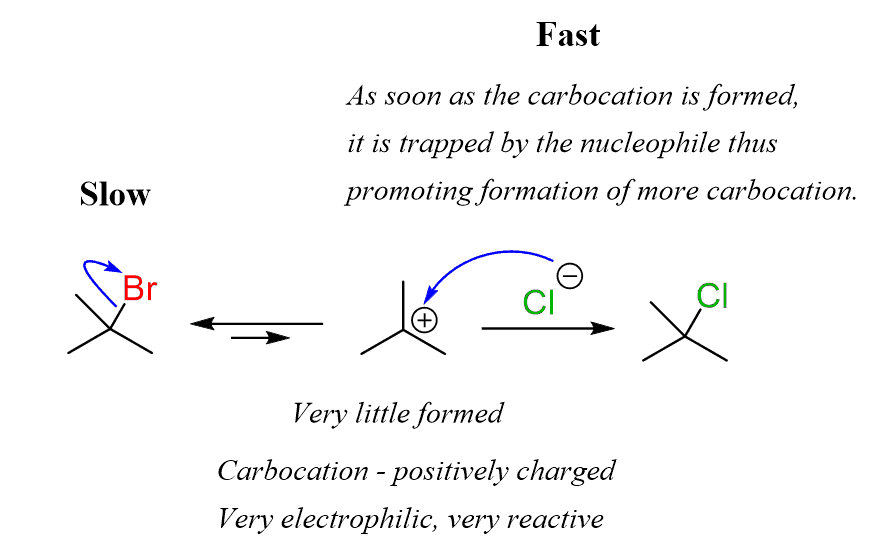
The fast reaction of the carbocation with the nucleophile is the driving force of the SN1 reaction since it pulls the equilibrium to the right according to Le Châtelier’s principle.
SN1 – A Two-Step Mechanism
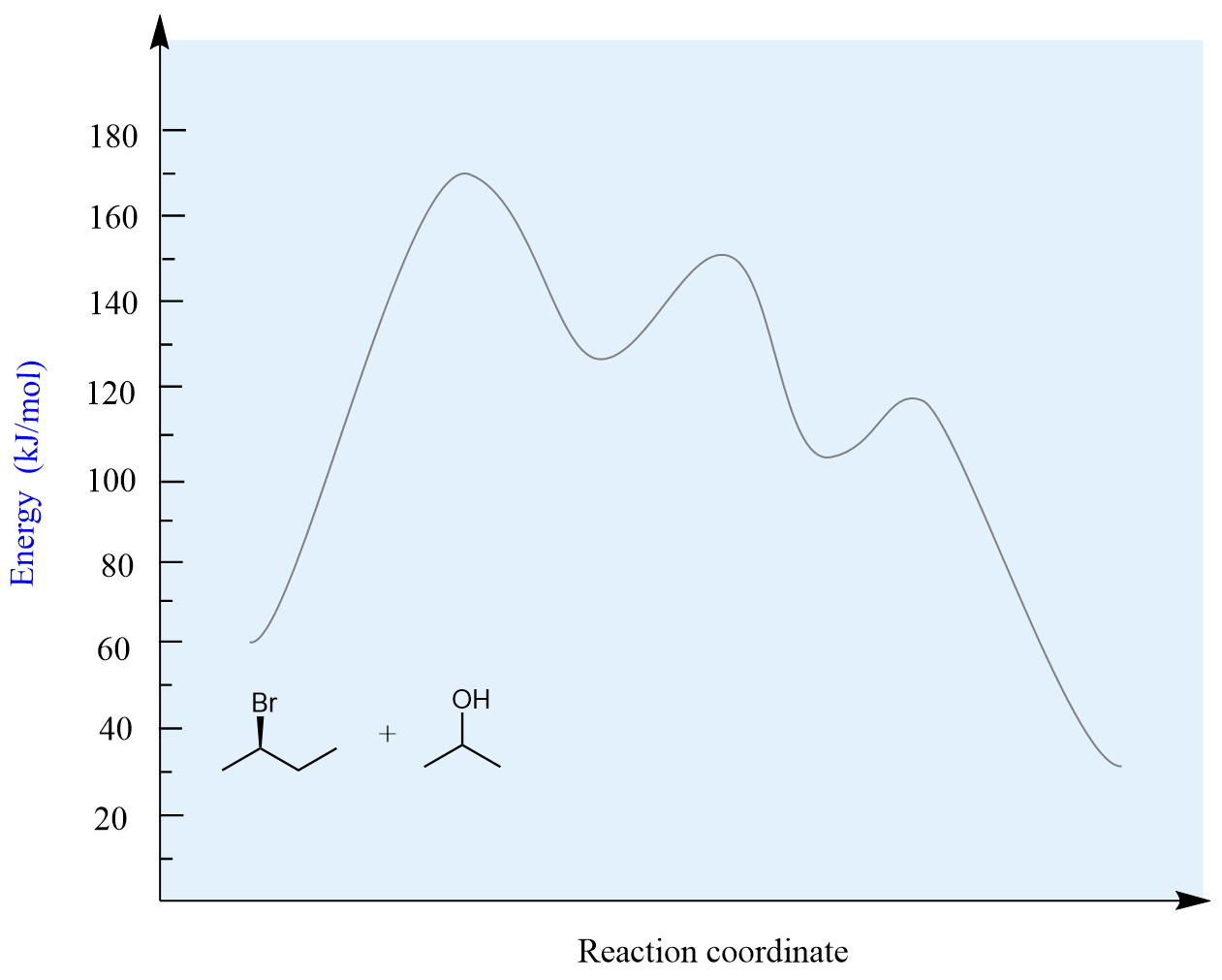
Let’s break down all the steps in the following SN1 reaction by looking at the energy diagram:
Step [1] Breaking the C–LG bond. In this rate-determining step, a carbocation intermediate is formed:

Step [2] A nucleophilic attack. The carbocation is highly electron-deficient, and the nucleophile attacks as a Lewis base using its lone pairs:

It is a two-step process; therefore, there are two transition states. Notice that the first activation energy is a greater/higher energy barrier to break the bond, which indicates a slower process. The carbocation is the intermediate of this reaction. Remember, the transition states are at the peak of the energy, and they are very unstable. The molecules exist in this state for only an instant, while the intermediates can exist for a significant time. However, compared to the reactants and products, carbocations are still very reactive, and they are attacked by the nucleophile as soon as formed.
Most often, substitution reactions are exothermic, and this is what is assumed for this example, too.
Let’s put what we covered so far about the SN1 reaction in a little summary and move on to other features such as the stereochemistry, reactivity of the substrate, and nucleophile:

Reactivity of Alkyl Halides in the SN1 Reaction
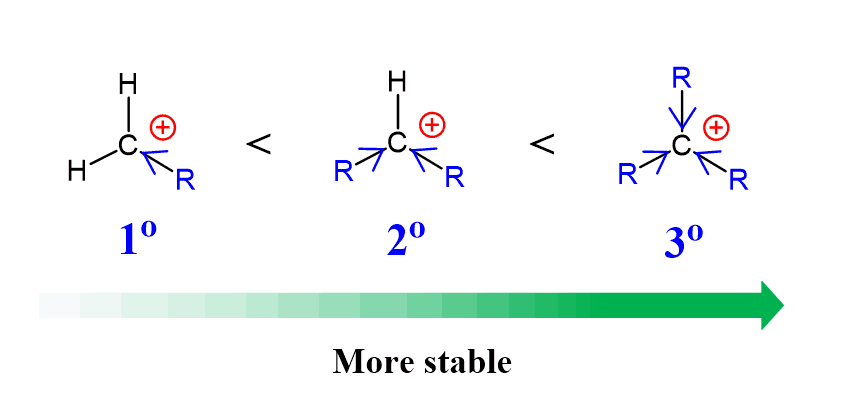
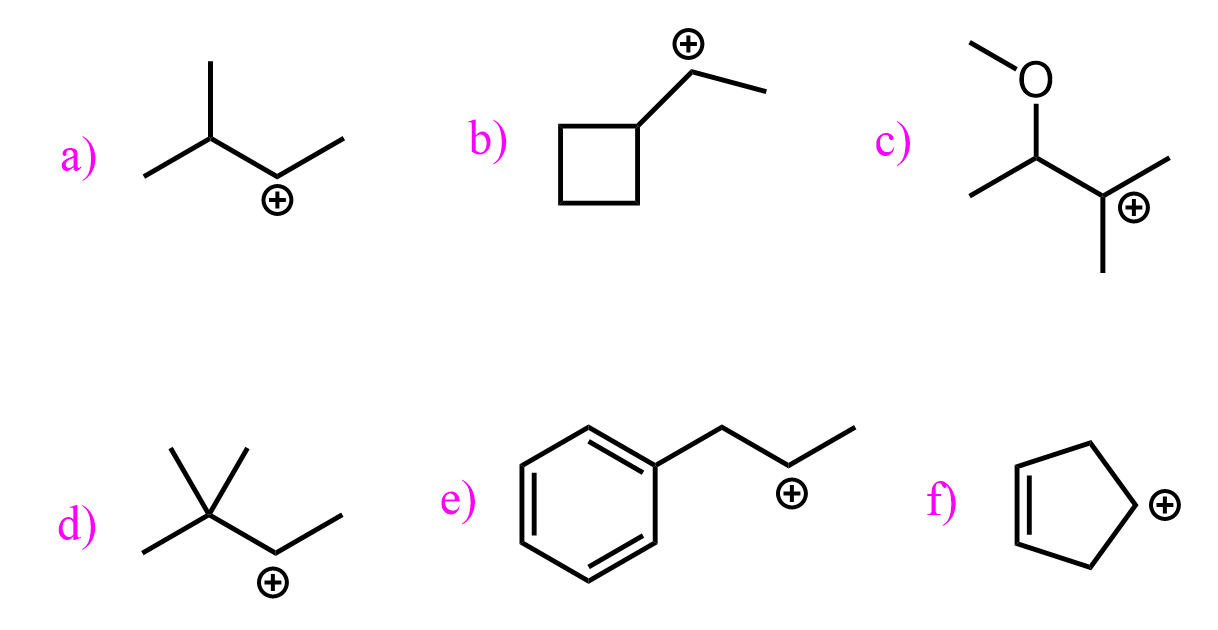
More substituted alkyl halides react faster in SN1 reactions:

The reason for this trend is the stability of the forming carbocations. And the reactivities in SN1 reactions indicate that the more substituted carbocations are more stable since the formation of the carbocation is the rate-determining step.
Why are more substituted carbocations more stable?
The reason is the electron-donating effect of alkyl groups and the hyperconjugation. The more alkyl groups connected to the carbocation, the greater the electron donation, thus stabilizing the positive charge. And this is the Inductive Effect:
What is Hyperconjugation?
Hyperconjugation is the charge stabilization by pushing some electron density of the adjacent σ bond to the empty p orbital of the carbocation. In the case of carbocations, where the sp2 carbon is surrounded by alkyl groups, the electron donation comes from an adjacent C-H bond. The more alkyl groups, the stronger the stabilizing effect of hyperconjugation.

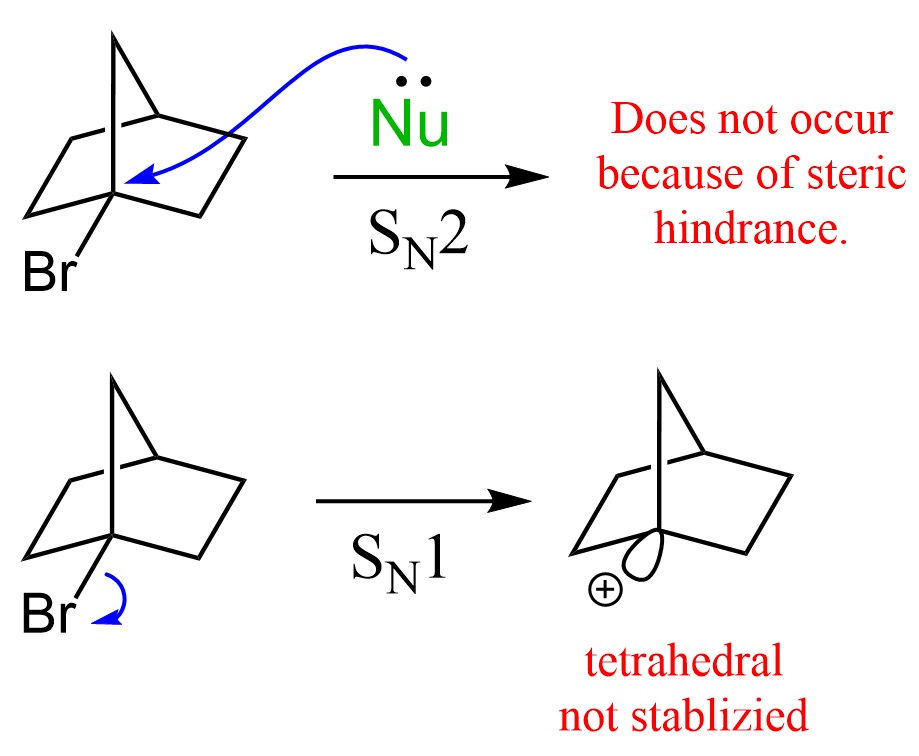
It does not have to be a C-H bond, but what is important is that the sp2 orbital is parallel with the electron-donating sigma bond. A great example illustrating this is the unreactive nature of bicyclic alkyl halides, where the carbon connected to the halogen is locked in a tetrahedral geometry. This means that it cannot be planar and thus stabilized by neighboring alkyl groups. For example, the following bicyclic alkyl halide does not react either by the SN1 (for the reasons we just mentioned) or SN2 mechanism because of the steric hindrance:
The Nucleophile in SN1 reactions
The nature of the nucleophile can often determine if the substitution goes through the SN1 or SN2 mechanism. This is a topic that deserves a separate article:
When Is the Mechanism SN1 or SN2?
However, for simplicity, remember that weak nucleophiles favor the SN1 while strong nucleophiles favor the SN2 mechanism:
And the good news is that there are just two types of common weak nucleophiles – water and alcohols.
What makes this a little challenging is the fact that both water and alcohols contain a hydrogen connected to the nucleophilic atom oxygen, and there is an additional acid-base step involved when these two are used as nucleophiles.
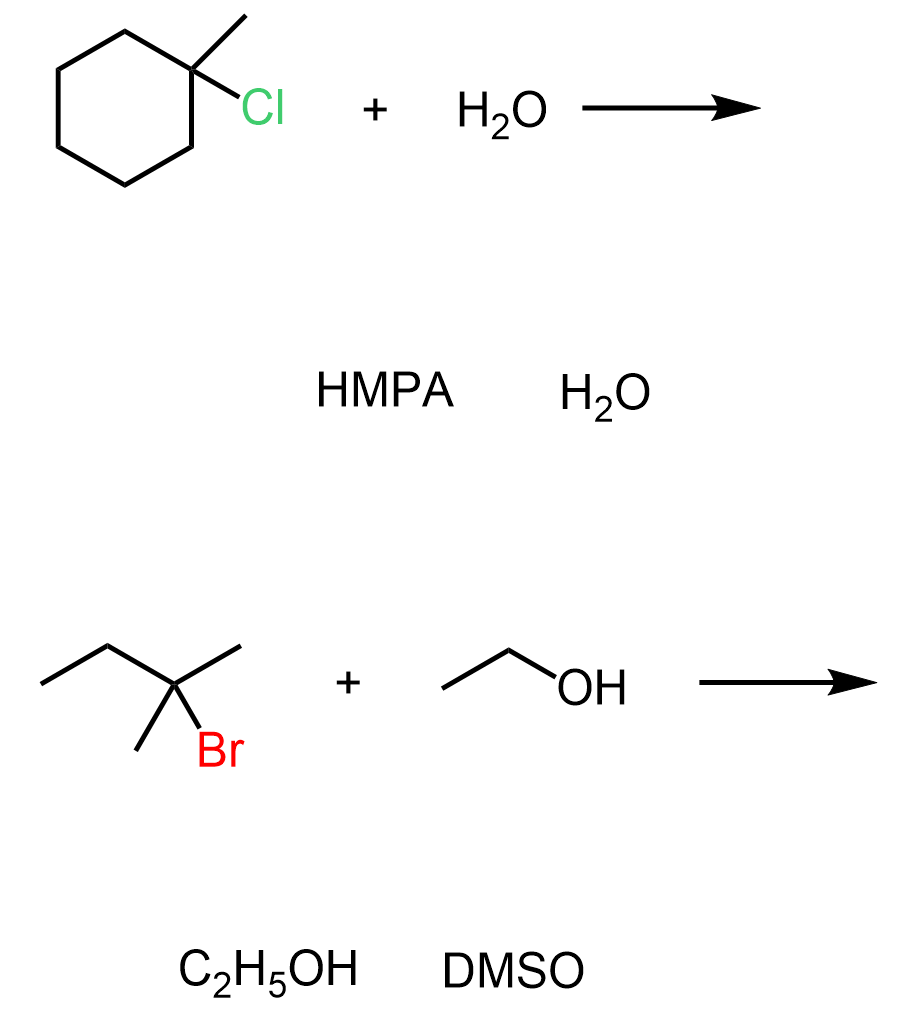
These SN1 reactions, and in any reactions where the solvent itself acts as the nucleophile, are also known as solvolysis.
Water and Alcohols as Nucleophiles in SN1 reactions
Let’s consider the following reaction between a tertiary alkyl halide and ethanol. Assuming an SN1 mechanism, draw the mechanism and the final product of this reaction:
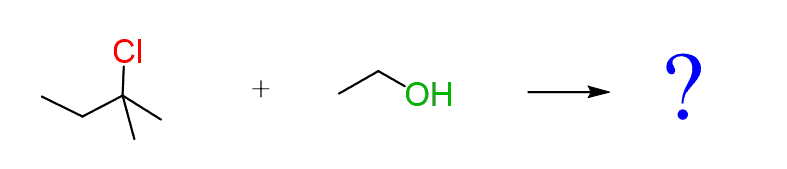
Remember the steps of SN1:
Step 1: Show the loss of the leaving group. Start the curved arrow from the middle of the bond and point it exactly to the leaving group:
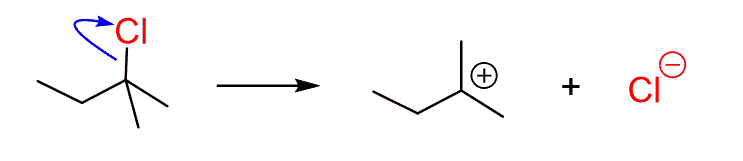
Step 2: Show the nucleophilic attack starting the curved arrow from a lone pair on the oxygen and point exactly at the carbocation:
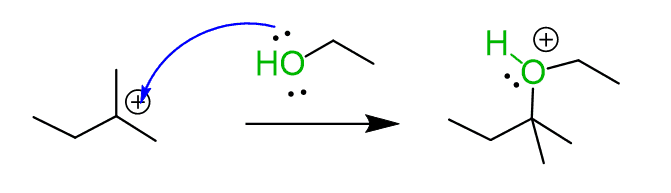
Step 3: Deprotonate the oxygen using the leaving group as a base in this acid-base reaction. The arrow starts from either a lone pair on the Br– or the negative charge. Breaking a bond is shown with an arrow starting from the middle of the bond and pointing to the leaving group:
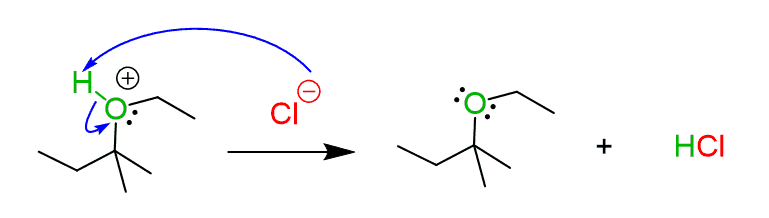
We just saw that the SN1 substitution of alkyl halides with alcohol produces an ether. The other most common weak nucleophile is water, and the product of these reactions is an alcohol. Check this article on the reaction of alkyl halides with water for more details and examples.
The Stereochemistry of SN1 reactions
Let’s determine the product of an SN1 reaction with a chiral substrate:
To do this, go ahead and draw the mechanism as we did earlier starting from the loss of the leaving group:

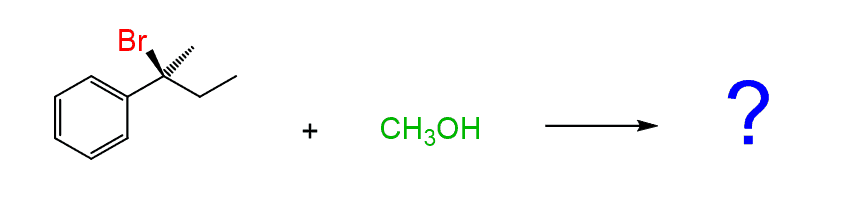
Now, before doing the nucleophilic attack, recall that carbocations are sp2-hybridized. The carbon has an empty p orbital perpendicular to the plane of the carbocation and the atoms connected to it:
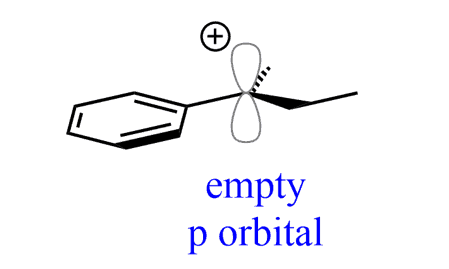
Notice that the loss of the leaving group generated a planar carbocation that is no longer chiral. And because of the planar geometry of the carbocation, the nucleophile can, and does, attack it from both sides because there is no preference for the attack from either direction:
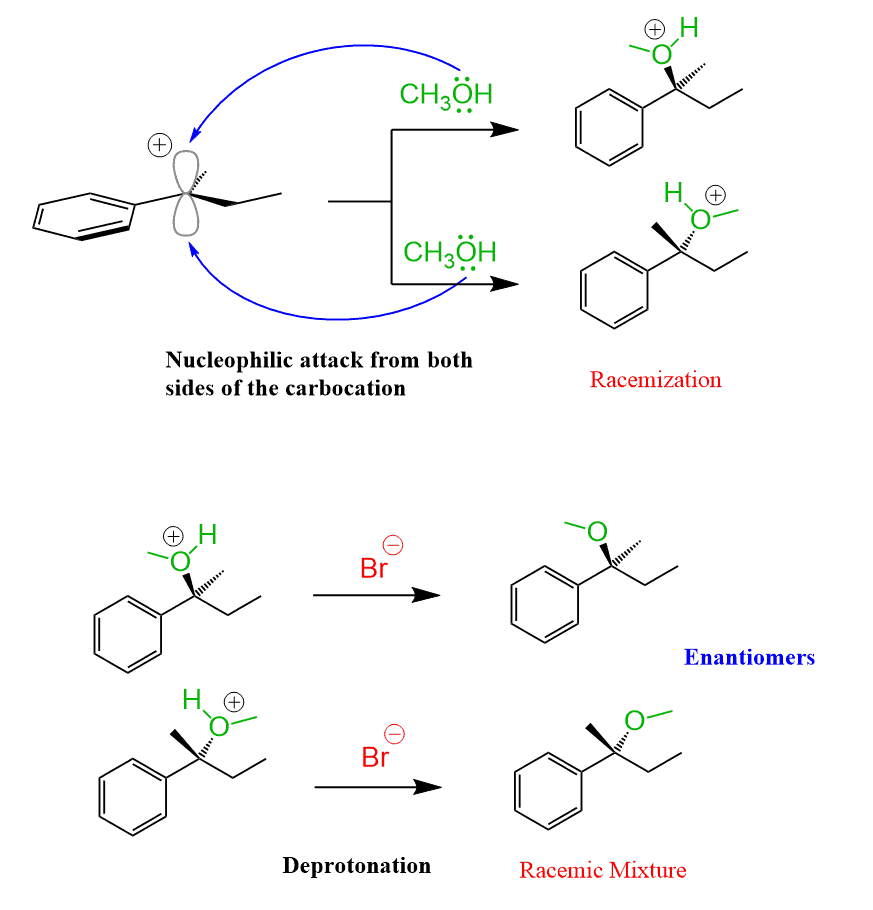
As a result, an equal amount of the two enantiomers (a racemic mixture) is formed. Therefore, SN1 reactions result in racemization.
This statement is true if the substrate contains only one chiral center, and this chiral center is part of the substitution reaction.
SN1 Reactions with more than one chiral center
For example, if the α-carbon is chiral and there is another stereogenic center in the molecule, a mixture of diastereomers is formed in a substitution reaction:
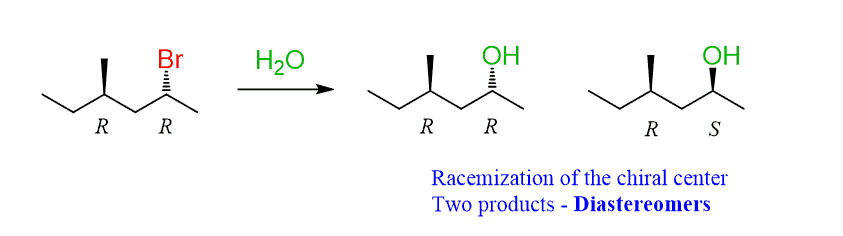
One of the chiral centers is inverted while the other one has the same configuration, therefore, these two are diastereomers.
So, to make the description of the stereochemistry of the SN1 reaction more accurate, we can say that:
SN1 reactions proceed with racemization at a single chirality center.
There is a separate post about the stereochemistry of SN1 reactions, so feel free to check that out as well.
Rearrangements in SN1 reactions
One feature of unimolecular reactions, such as SN1 and E1 is the rearrangements. This is when the nucleophile and the leaving group appear on different carbon atoms:
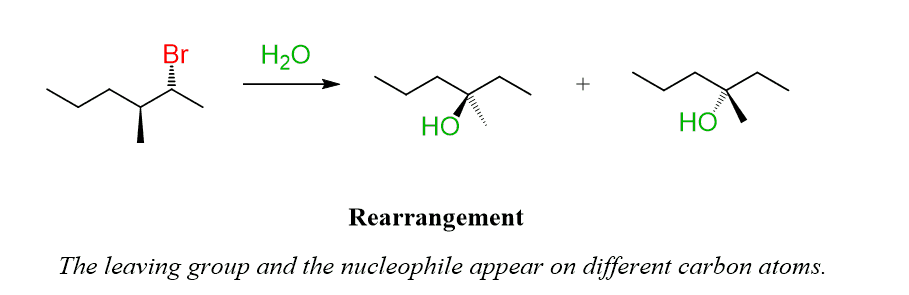
The reason for this “misplacement” is the fact that carbocations tend to undergo rearrangements to transform into more stable ones. This can happen either by a 1,2-hydride shift or a 1,2-methyl shift.
And because SN1 goes through the formation of a carbocation, it does involve a rearrangement whenever a more stable carbocation can be generated. This is covered in more detail in the following post: “Carbocation Rearrangements in SN1 Reactions with Practice Problems”
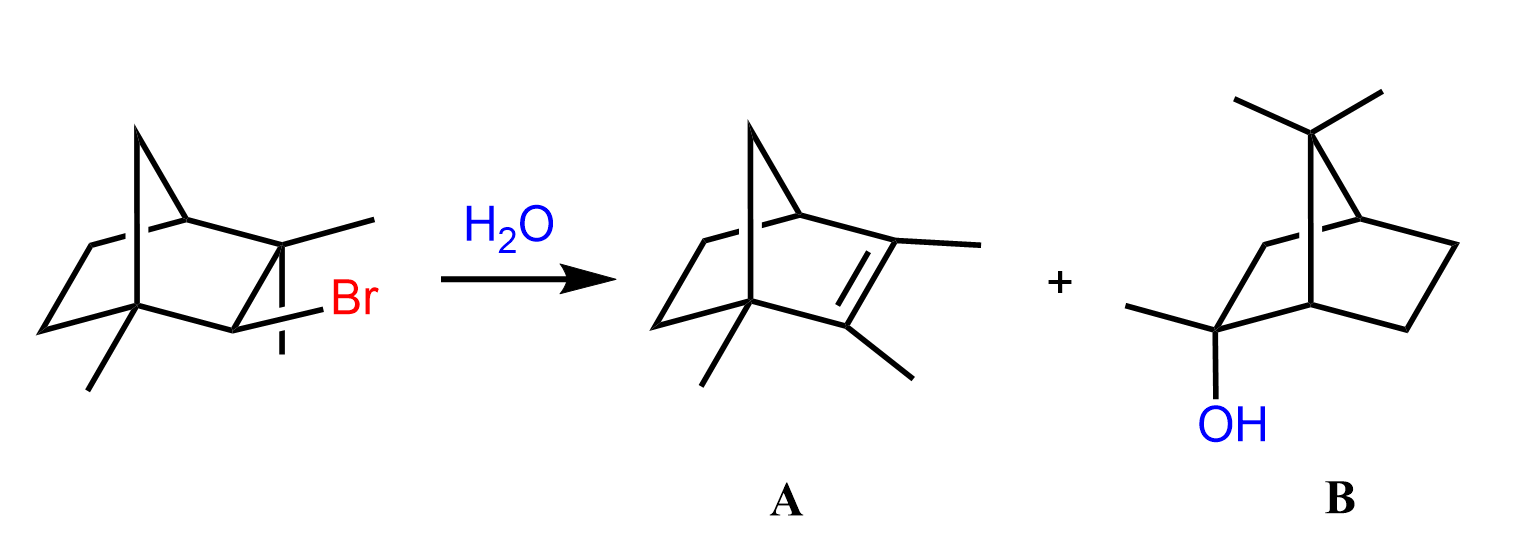
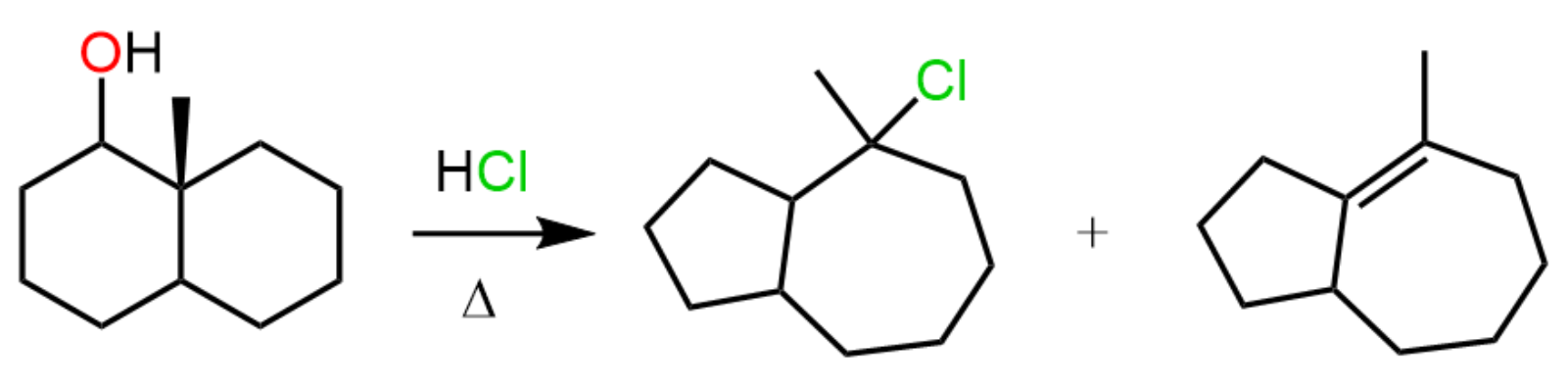
Another type of rearrangement that can occur in SN1 reactions is the ring expansion rearrangement, where a smaller ring is converted into a larger ring as a result of a hydride or alkyl shift:
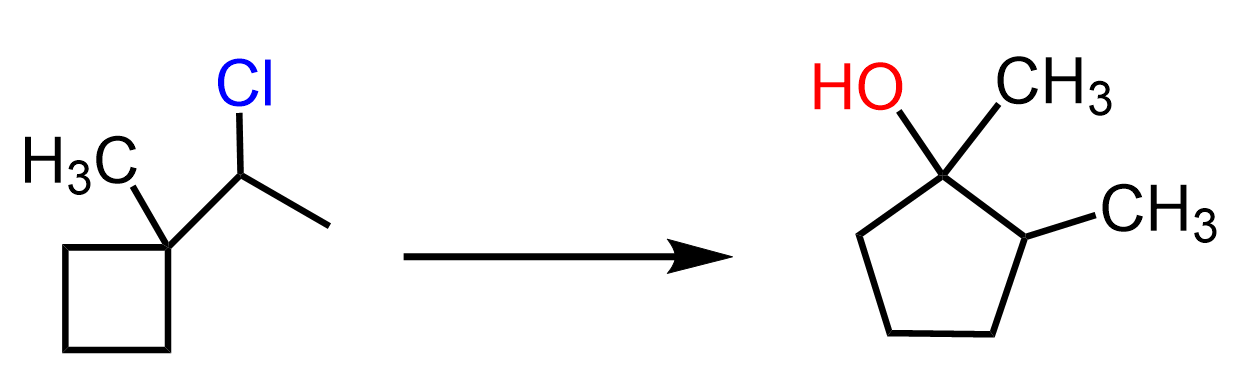
This has been a long post with a lot of information, so let’s keep the ring expansion rearrangements for another one, especially since there are also ring-contraction rearrangements.
Below are some more practice problems on SN1 reactions and a multiple-choice quiz that covers SN1, SN2, E1, and E2 reactions.









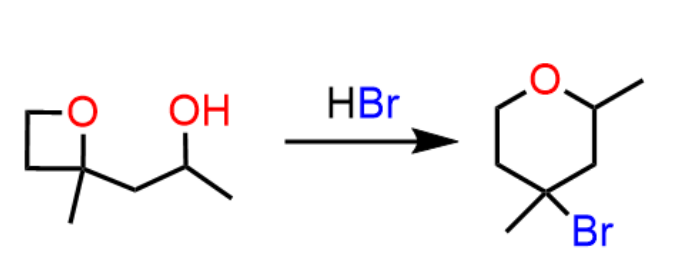
Hi Professor: I was working on what seemed to be a simple SN1 reaction of water and 5-bromo-2-methyl-hexan-2-ene. The bromine is obviously the leaving group, but since carbocations can rearrange, would we get a hydride shift putting the positive charge on the neighboring allylic ion and yielding two products, 2-methyl-2-hexan-2-ene-3-ol (with enantiomers) and the alcohol on the tertiary allylic carbon (through resonance) 2-methyl-hexan-3-ene-2-ol. Am i overthinking this?
Hi there,
You are not overthinking as this question requires considering a lot of possibilities. Everything you mentioned is correct and the major product is likely (I do not have experimental data) 2-methylhex-3-en-2-ol which forms via the more stable tertiary allylic carbocation. E1 elimination is also possible here because the resulting diene is conjugated and one of the double bonds trisubstituted. This would have a greater contribution with increasing temperature as heat favors elimination.