Alkyl halides are molecules in which a halogen is bonded to an sp3 hybridized carbon atom. They are classified into Primary, Secondary, and Tertiary Alkyl Halides, which are especially important in nucleophilic substitution and elimination reactions.
To distinguish between a primary, secondary, or a tertiary alkyl halide, locate the carbon that is connected to the halogen and count how many carbon atoms are connected to it:

Other common halides in organic chemistry are the aryl– and vinyl halides:

Both in vinyl halides and aryl halides, the halogen atom is connected to a carbon-carbon double bond, and they do not undergo nucleophilic substitution reactions, which we are going to discuss in this chapter.
In short, substitution reactions occur when alkyl halides are treated with nucleophiles, and elimination reactions occur when they are reacted with bases.
The nucleophile replaces the halide, while in elimination reactions, the base removes a proton together with the halide, thus making a new π bond:
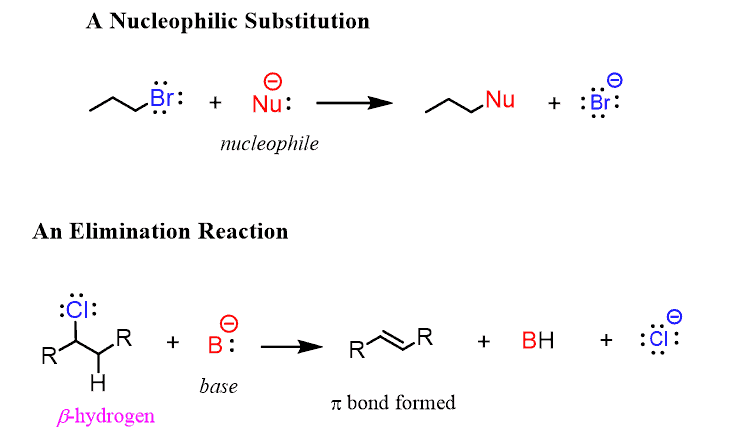
The reactivity of alkyl halides in substitution reactions is explained by the imbalance of the electron density between the carbon and halogen (leaving group). It is a polar covalent bond where the more electronegative halogen pulls the electron density, thus making the carbon partially positively charged:
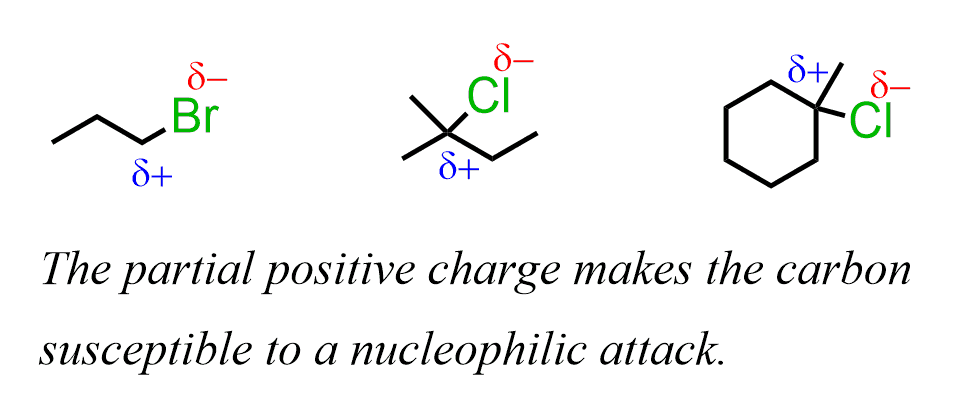
The carbon is, therefore, electrophilic – it is looking for some electron density to compensate for the partial charge. And, species with a high electron density are going to attack this carbon.
The molecule with the leaving group and the electrophilic carbon is called an electrophile. The most common electrophiles are the alkyl halides since the Cl–, Br–, and I– are good leaving groups.
Just like we call the carbon electrophilic, the species with a high electron density is called a nucleophile – “loving nucleolus”, i.e., loving a positive charge.
Allylic halides are sort of in between the alkyl halides and aryl halides. The allylic position is the sp3-hybridized carbon atom connected to the one in a double bond:

When the double bond is part of an aromatic system, then we have benzylic halides. These two are very reactive and, unlike vinylic halides, they do undergo substitution and elimination reactions.

There are two main mechanisms for nucleophilic substitution and elimination reactions: SN1, SN2, E1, and E2. Each of these needs at least one separate post to cover all the details, and you can find the links to them below.
Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:
Nucleophilic Substitution and Elimination Practice Quiz
Check Also
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
Nucleophilic Substitution Reactions
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Is it SN1, SN2, E1 or E2 Mechanism With the Largest Collection of Practice Problems
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Introduction to Substitution Reactions
- All You Need to Know About the SN2 Reaction Mechanism
- The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- Mechanism and Stereochemistry of SN2 Reactions with Practice Problems
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- The SN1 Nucleophilic Substitution Reaction
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- The Role of the Solvent in SN1, SN2, E1 and E2 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- When Is the Mechanism SN1 or SN2?
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Alcohols in Substitution Reactions with Tons of Practice Problems
Elimination Reactions
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Is it SN1, SN2, E1 or E2 Mechanism With the Largest Collection of Practice Problems
- General Features of Elimination
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- Dehydration of Alcohols by E1 and E2 Elimination
- Stereoselectivity of E1 Reactions
- Nucleophilic Substitution vs Elimination Reactions
- Regioselectivity of E1 Reactions
- E2 vs. E1 Elimination Mechanism with Practice Problems


good and nice