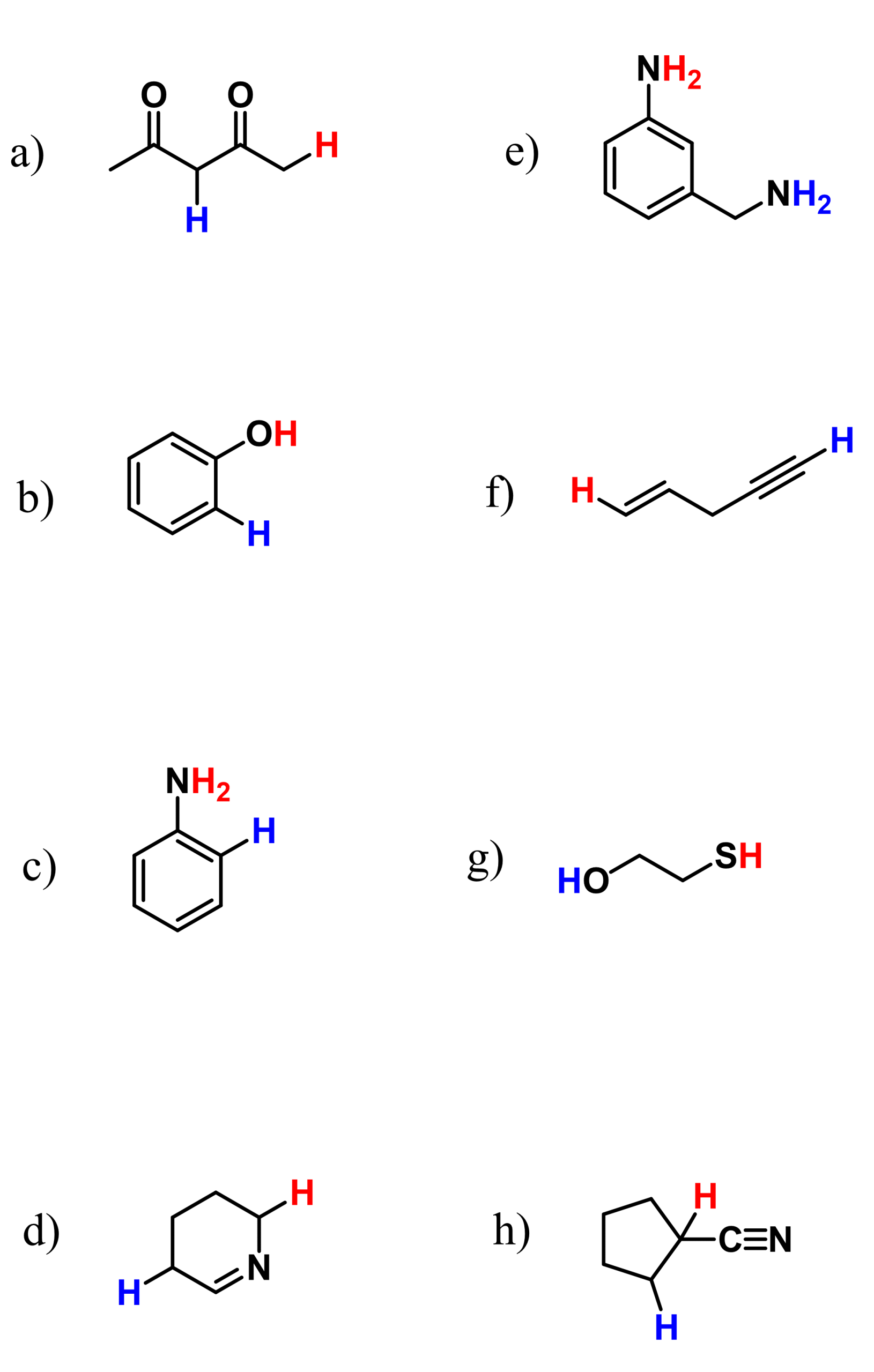
In the previous post, we talked about the acid strength and its quantitative description by pKa. The pKa table shows how greatly the acidity varies for different functional groups. For example,

The question is, how do we explain these differences? Well, remember, we said that the stronger the acid, the weaker its conjugate base. For example,

And a weaker conjugate base means a more stable conjugate base because if it were not as stable as it is, it would have reacted with the proton and shifted the reaction backward, forming the acid.
Therefore, the explanation of the acidity relies on the stability of the conjugate base. There are four main factors, in the following priority order, that affect the stability of the conjugate base:
- Atom
- Resonance
- Induction
- Orbital
This is often abbreviated and referred to as ARIO or CARIO.
Let’s go over those one by one:
The Atom and Acidity
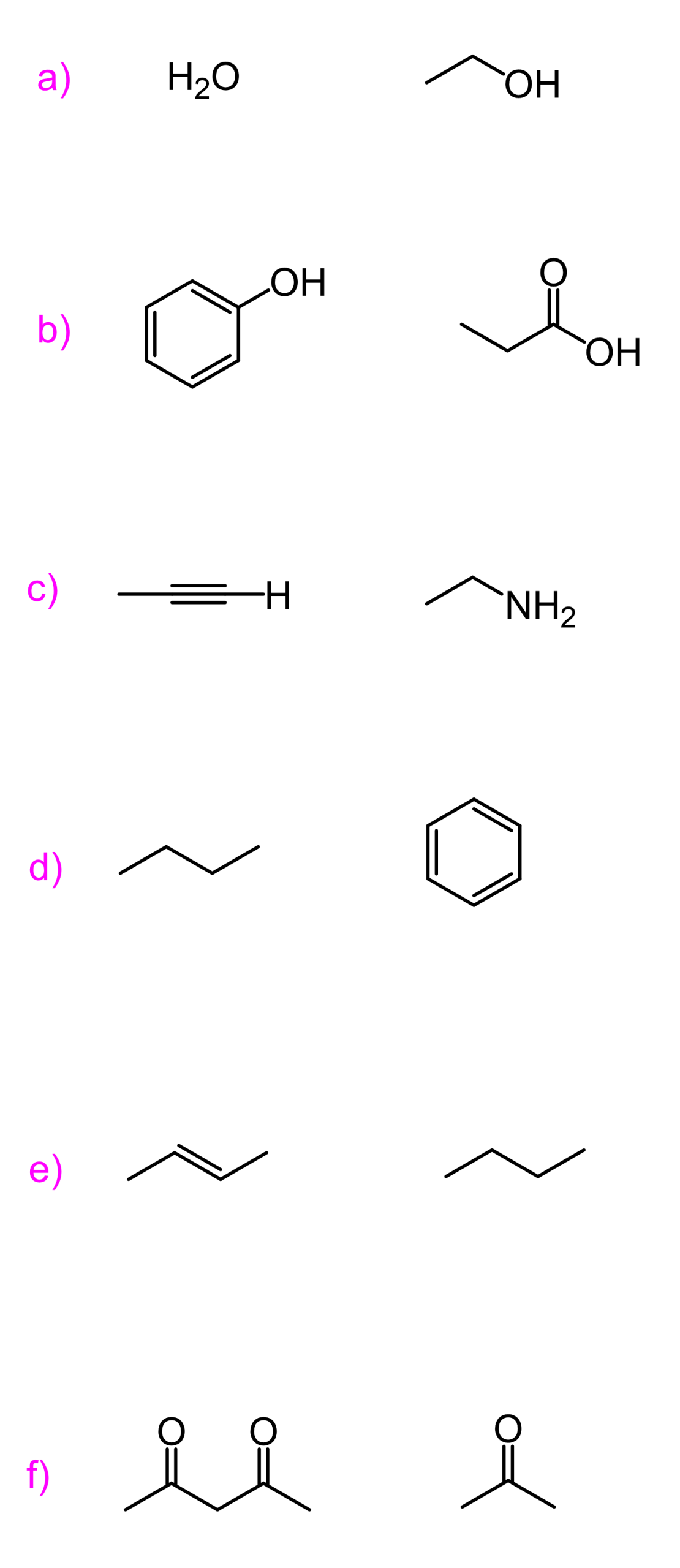
Here, we are talking about the atom that is connected to the hydrogen. The better it stabilizes the negative charge, the more stable the conjugate base is. For example, we know that alcohols are more acidic than amines:

And this means that the oxygen stabilizes the negative charge better than the nitrogen.
How do you think it does that?
The answer is that it is more electronegative than nitrogen – it likes electrons/negative charge or doesn’t mind them as much as nitrogen does.
So, remember the first factor is the atom connected to the H, specifically the electronegativity of this atom.
The acidity of H–A increases as the electronegativity of A increases, going from left to right in the periodic table:

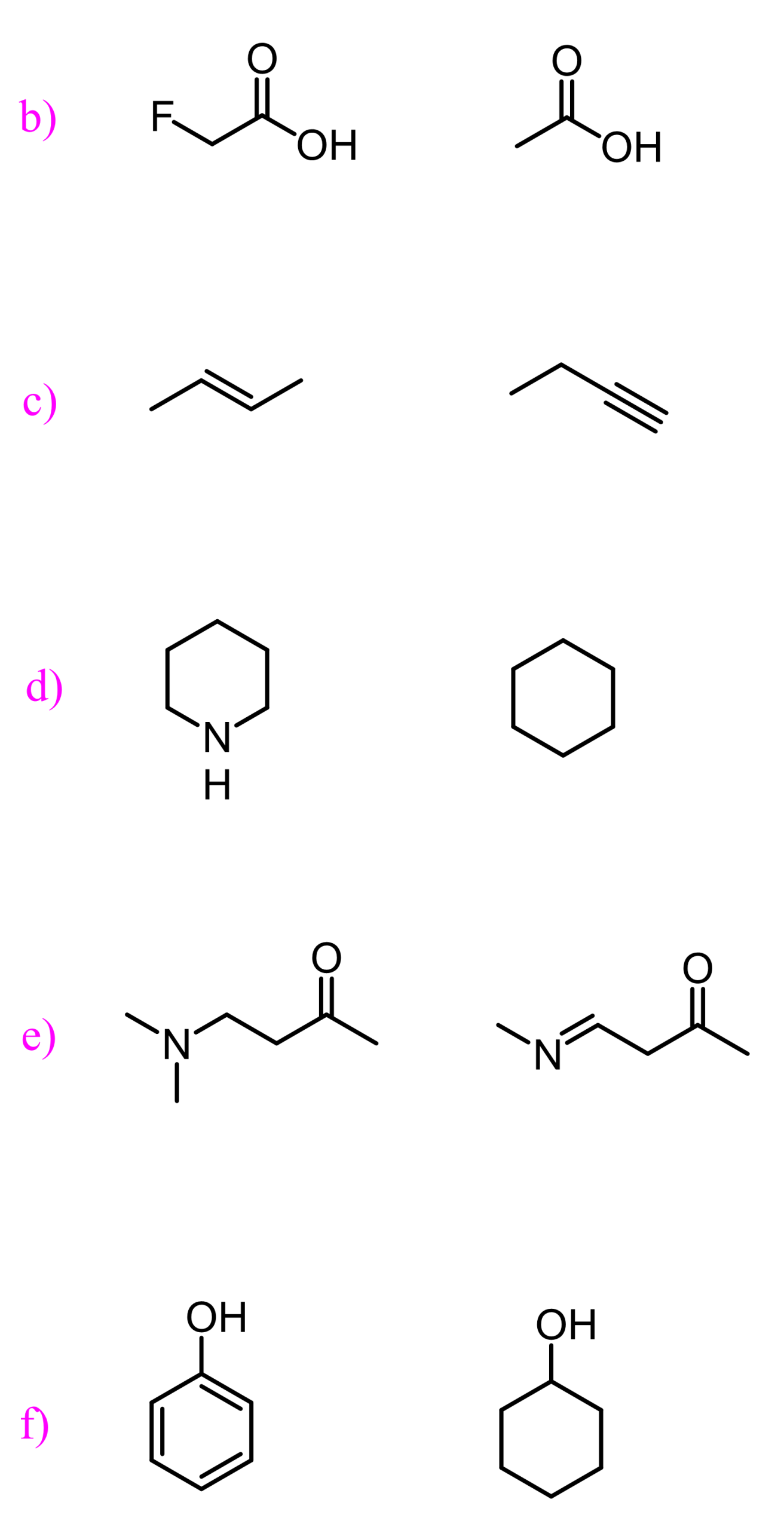
Atomic size and Acidity
If you look in the pKa table, you will see that thiols (R-SH) are more acidic than alcohols:

This is interesting because oxygen is more electronegative than sulfur, and you’d expect the opposite acidity trend. The reason for this is the ability of larger atoms to better stabilize the negative charge:

You can think about it this way: the negative charge is spread around a larger surface/volume, thus it is better stabilized.
Therefore, down the periodic table, the atomic size determines the acidity and not the electronegativity.
Resonance and Acidity
Let’s write the dissociation equation for an alcohol and a carboxylic acid and see why carboxylic acids are more than a billion times more acidic than alcohols:
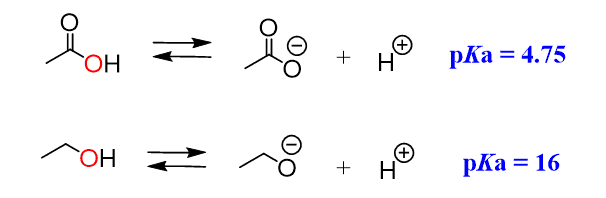
From what we have learned so far, we need to look at the atom connected to the H and, in both cases, it is an oxygen. So, the first factor, the atom, does not explain this big difference in the acidity.
If you have already realized that it is a result of resonance stabilization, great job!
The electrons on the oxygens of carboxylic acid are delocalized and the negative charge is handled by both atoms, while the oxygen in the alcohol handles the negative charge alone:
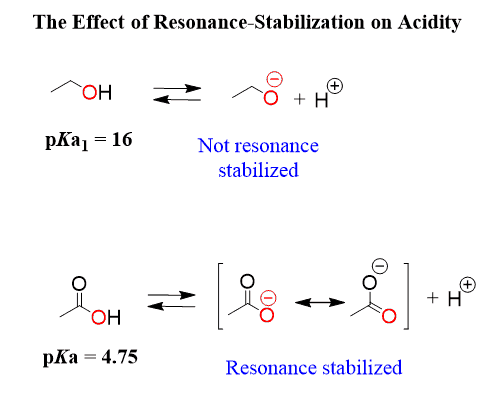
We have a more detailed discussion about the effect of resonance on the acidity and basicity of in this article, so feel free to check that out as well.
Induction and Acidity
How do we explain the difference in the acidity of the following two carboxylic acids?

The electronegativity and the resonance stabilization do not explain this difference as in both molecules we are comparing a resonance-stabilized oxygen atom.
One thing we remember about fluorine is that it is the most electronegative element. So, the question is how the presence of three electronegative atoms increases the acidity of the O-H bond.
And the answer to this is that the electronegativity of the fluorine remotely helps the oxygen to handle the negative charge. They are pulling some of the electron density, thus reducing it on the oxygen:
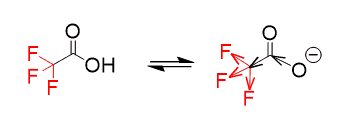
This is called the inductive effect.
The stronger the electronegativity, the stronger the inductive effect:

Another factor is the distance of the electronegative element from the negative charge. The closer it is, the better it helps to stabilize the negative charge:

Orbital/Hybridization and Acidity
To illustrate this concept, we will consider the acidity of hydrocarbons:
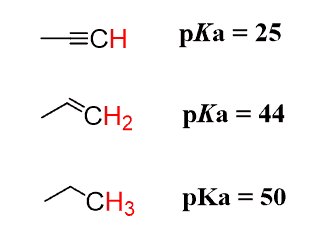
None of the factors discussed earlier can be used to explain this trend of acidity. We have the same atom (C), no resonance stabilization, and no inductive effect.
Let’s put a negative charge on each carbon and take a closer look:

The only difference is that these carbons are in their hybridization state. Remember, alkanes are sp3, alkenes are sp2, and alkynes are sp-hybridized.
What you need to know is that s orbitals are more electronegative than p orbitals, and the more s character the hybrid orbital has, the better it stabilizes the negative charge. These are the percentages of the s orbital (s-character) in each hybrid orbital:

To summarize, whenever you need to determine the more acidic proton, visualize (or better draw) the conjugate bases and determine which one is the more stable base on the ARIO factors.
- Once again, check this article for a more detailed discussion about the resonance and inductive effects on acidity and basicity.
- Here is also a pKa table for the most common functional groups in organic chemistry, and you can find the more comprehensive version in the linked article.