In the previous post, we talked about the resonance structures and the main rules applied to them.
In summary, there are two must-follow rules when drawing resonance structures:
1) Do not exceed the octet on 2nd-row elements.
2) Do not break single bonds
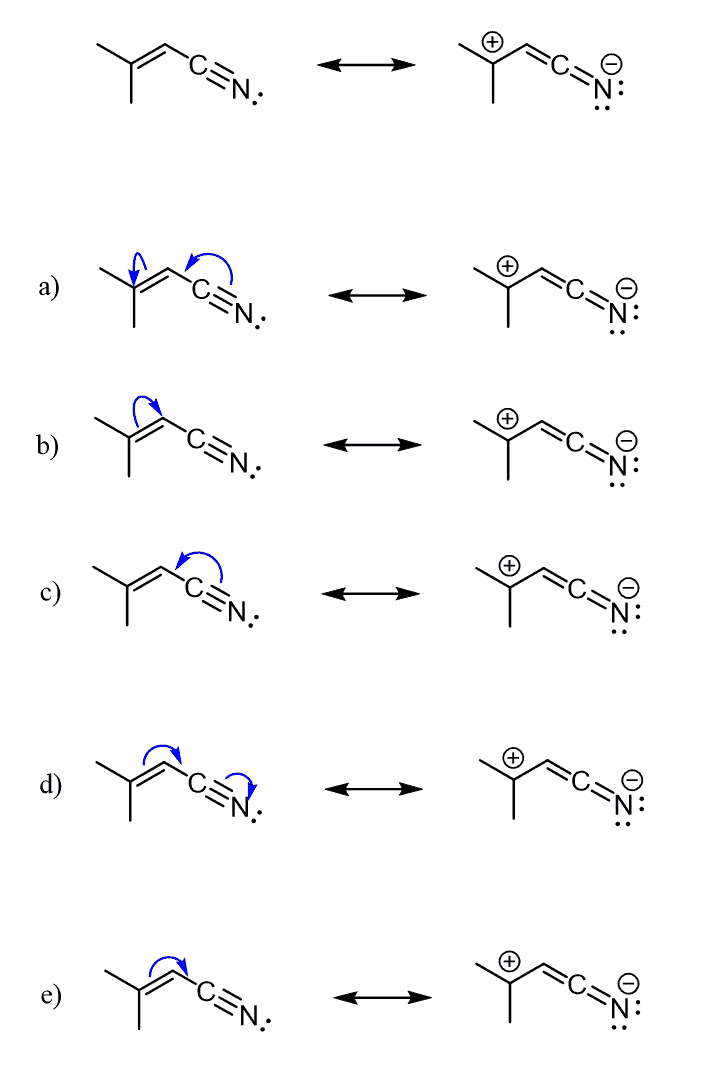
Keeping these in mind, go ahead and work on the following practice problems on drawing curved arrows, missing resonance forms, and determining the more stable resonance structure.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance Structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.







If I pass my organic chemistry exam tomorrow, it will be thanks to you!! Found this page way too late!
Good luck with the exam. Let us know!