Lone Pairs and Resonance Stabilization
Before we classify the lone pairs of electrons as localized or delocalized, let’s answer a quick question about resonance structures: Which of the following represents a correct transformation between the two resonance structures?

If your answer is the first transformation, then great.
If it is not, or you are not sure how to answer this question, remember that resonance structures are two Lewis structures of the same compound, meaning that all the atoms have the same connectivity/ placement (connected to the same neighboring atoms) and they differ only by the arrangement of electrons. Because of this, when drawing resonance transformations, we follow these two rules:
1) Not breaking a single bond, and
2) Not exceeding the octet on second-row elements
Therefore, if we were to move the second lone pair of electrons as shown in the equation, we would have exceeded the octet on the carbon next to it, and this is something you never want to do. If you cannot spot the carbons with exceeding octet immediately, add the invisible hydrogens on the bond-line structure:

As you can see, the carbon with two hydrogen atoms has five bonds (10 electrons), and this is why the lone pairs on the nitrogen cannot participate in resonance stabilization – they are localized.
Localized and Delocalized Lone Pairs
Now, leaving aside the chemical terminology, in simpler words, one pair of electrons can move around, while the other pair cannot. These electrons belong to only one atom – they are localized. The ones that can move around are delocalized – they can be placed on one atom, but they can also be shared between that and the neighboring atom, i.e., can participate in resonance stabilization.
In a similar way, the same element in one molecule can have localized and delocalized lone pairs of electrons. As an example, the two oxygens of an ester group possess localized and delocalized lone pairs.

The red electrons on the oxygen can participate in resonance stabilization because of the possibility of moving up the π bond electrons. Therefore, these are delocalized electrons. The blue electrons, on the other hand, are localized on the top oxygen because the only way of moving them down would be either exceeding the octet of the carbon (this really means there is no way) or breaking the single bond between the carbon and the other oxygen, which again, goes against the rules of resonance structures.
All these examples of localized and delocalized lone pairs show when lone pairs can and cannot be involved in resonance transformations. See this article for more details and a simple set of rules for drawing resonance structures.
Hybridization and Delocalization
Now that we have learned how to classify electrons as localized or delocalized, let’s understand the geometry of the elements participating in delocalization. One way to visualize delocalization is that electrons flow through the orbitals of adjacent atoms. These electrons can be non-bonding (lone pairs) or bonding electrons. More specifically, they must be either non-bonding or π-bond electrons. When the electrons in π bonds are capable of flowing through the orbitals of adjacent atoms, they are said to be in a conjugated system.
For example, if the molecule contains two bonds, then it is a diene (two alkenes – two “enes”), and dienes can be conjugated, isolated, or cumulated:

You can read more about conjugated systems here; however, in this article, we will focus on localized and delocalized electrons. Now, the reason I mentioned dienes and conjugated systems is that you need to remember that for the electrons to be delocalized, they must be in parallel p orbitals!
For example, in butadiene, the overlapping p orbitals on adjacent atoms allow the electrons to be delocalized over the four or more atoms. Once again, to achieve this delocalization, all the p orbitals must be aligned in parallel:

This requirement restricts the delocalization to atoms that are either sp2 or sp3 hybridized because in sp3 hybridization, there is no p orbital by itself – all the p orbitals are mixed with the s orbital.
Now, considering this, what would you think the hybridization of the nitrogen next to the double bond is?

On one hand, it looks to be sp3 since it only has single bonds, the steric number is 4, indicating a tetrahedral geometry.
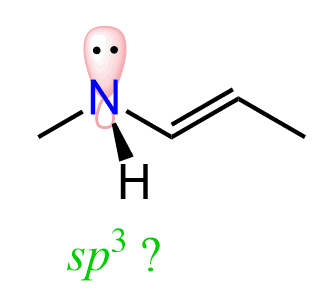
However, we now know that the lone pair is delocalized because of the possible resonance structure. And in order to be delocalized, this lone pair must be in a p orbital, which is parallel to the p orbitals of the double bond. Therefore, the hybridization of the nitrogen is sp2.

This is a general trend to remember: atoms next to a π bond are sp2-hybridized, which enables resonance delocalization of the lone pair with the π bond electrons.
For example, the hybridization of the nitrogen in amides is also sp2, even though structurally it may look like an sp3 atom.
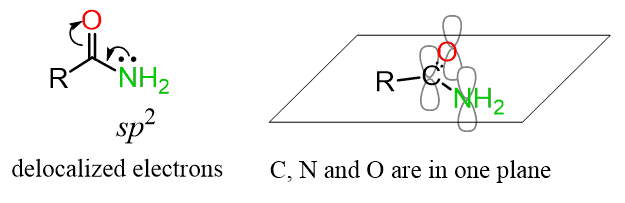
I’ve also written a separate post on how lone pairs affect hybridization, so check it out for more details and examples!
To summarize, when you are asked to determine whether the lone pairs are localized or delocalized, you need to check which ones can be involved in resonance transformations and which cannot. The lone pairs next to π bonds are delocalized because they are in the p orbital of an sp2 hybridized atom.
If the lone pairs can participate in forming resonance contributors, they are delocalized; if the lone pairs cannot participate in resonance, they are localized.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.
Molecular Representations Quiz



Very supportive