Nitration of benzene or its derivatives introduces a nitro (NO2) group into the aromatic system. The reaction is carried out with a mixture of nitric and sulfuric acids at temperatures above 50 oC.
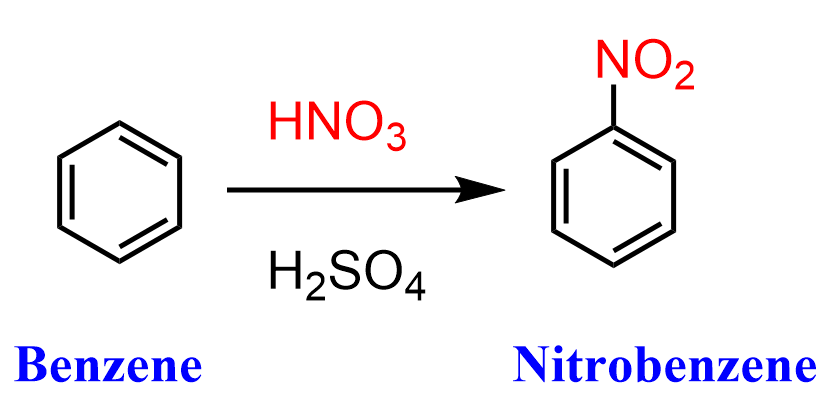
The role of sulfuric acid here is to create the nitronium ion (+NO2), which is the immediate electrophile reacting with the aromatic ring. The nitronium ion is formed as a result of the protonation of the nitric acid by sulfuric acid:
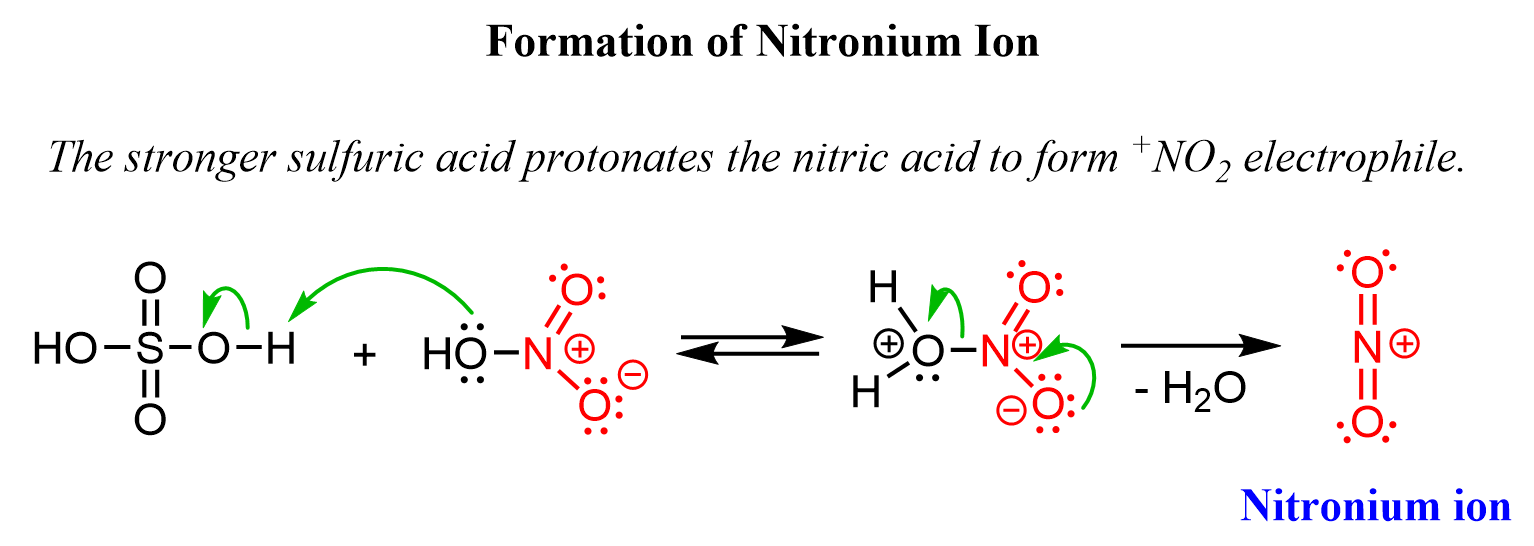
Once the nitronium ion is formed, it is attacked by the electrons of the aromatic system according to a standard electrophilic aromatic substitution mechanism.

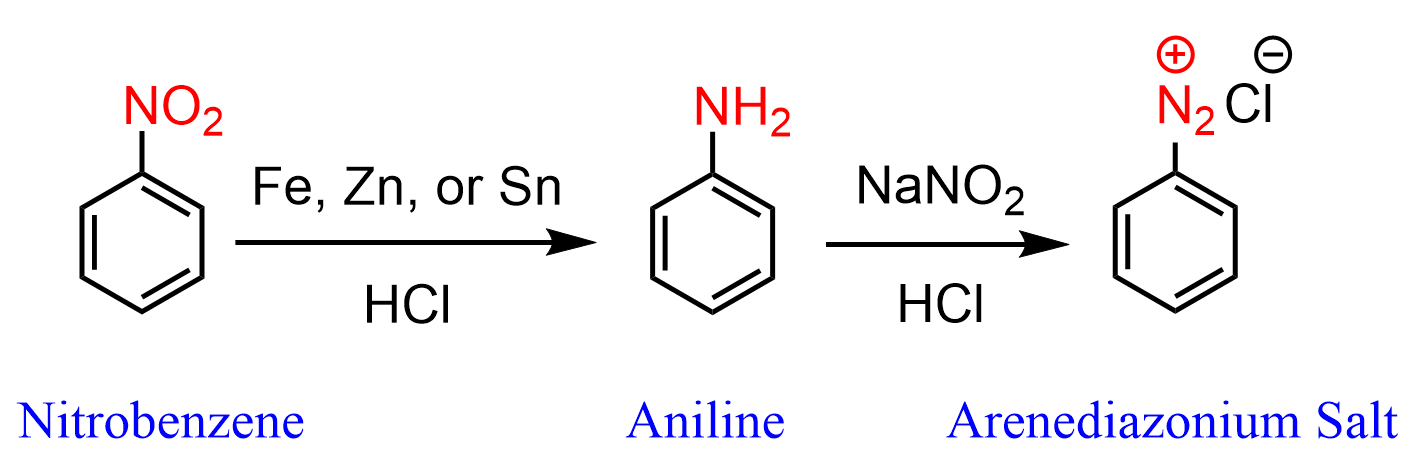
Nitration of benzene is a very important reaction as it produces a source for synthesizing other nitrogen derivatives such as aniline, which, in turn, can undergo a variety of electrophilic aromatic substitutions, act as a base or nucleophile, and be used for making arene diazonium salts:
How does the Nitro Group Affect the Reactivity of Benzene?
You may not have covered this yet, but all the groups connected to the benzene ring are classified as activators and deactivators. Activators are those that make electrophilic substitution reactions (EAS) faster/easier compared to benzene, and deactivators are those that slow down or make certain EAS reactions impossible. Most activating groups are ortho, para, and deactivating groups are meta directors:

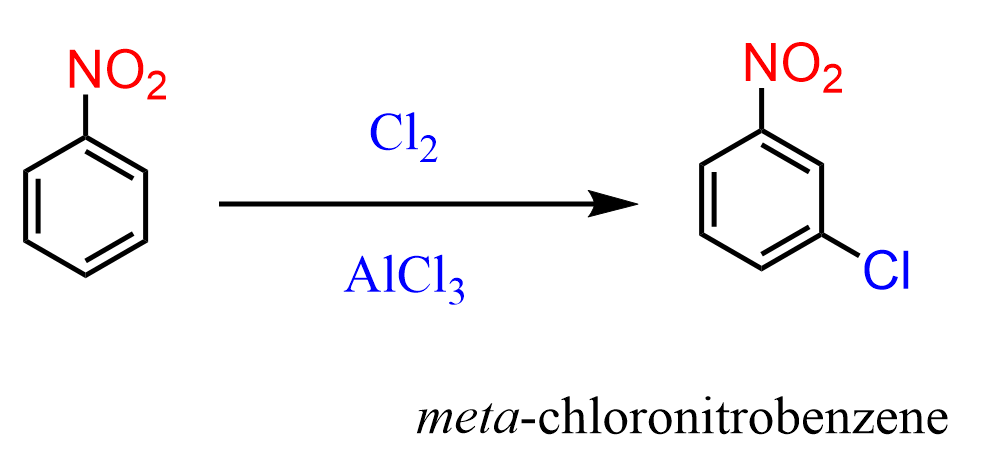
The nitro group is a strong deactivator and thus a meta director, so it slows down the electrophilic aromatic substitutions. For example, the halogenation of nitrobenzene requires temperatures above 100 oC.
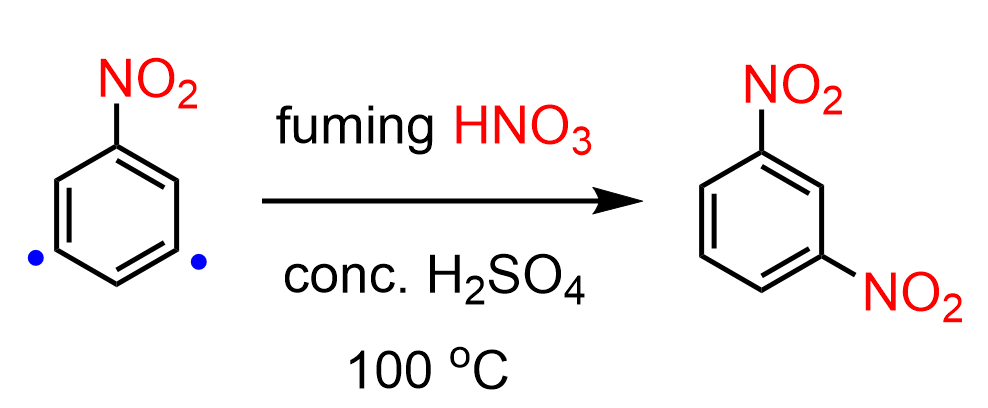
A question you may be wondering about is whether nitrobenzene can be further nitrated. The answer is yes, we can add two nitro groups to benzene; however, the nitration of nitrobenzene requires stronger conditions because the aromatic ring is now deactivated. The temperature is raised to 100 °C, and fuming nitric acid is used instead of normal concentrated nitric acid:
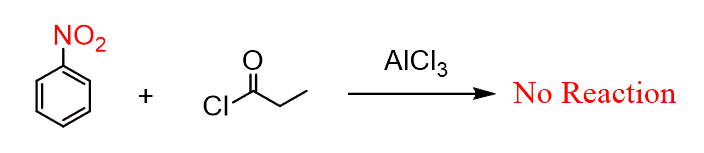
The deactivating effect prevents certain EAS reactions from occurring. For example, Friedel-Crafts alkylation and acylation reactions are among the slowest in electrophilic aromatic substitutions, and they simply do not work for nitrobenzene:
This is by no means the only limitation in electrophilic aromatic substitution. Check out this post for more examples and strategies to overcome common challenges in EAS.”
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds
