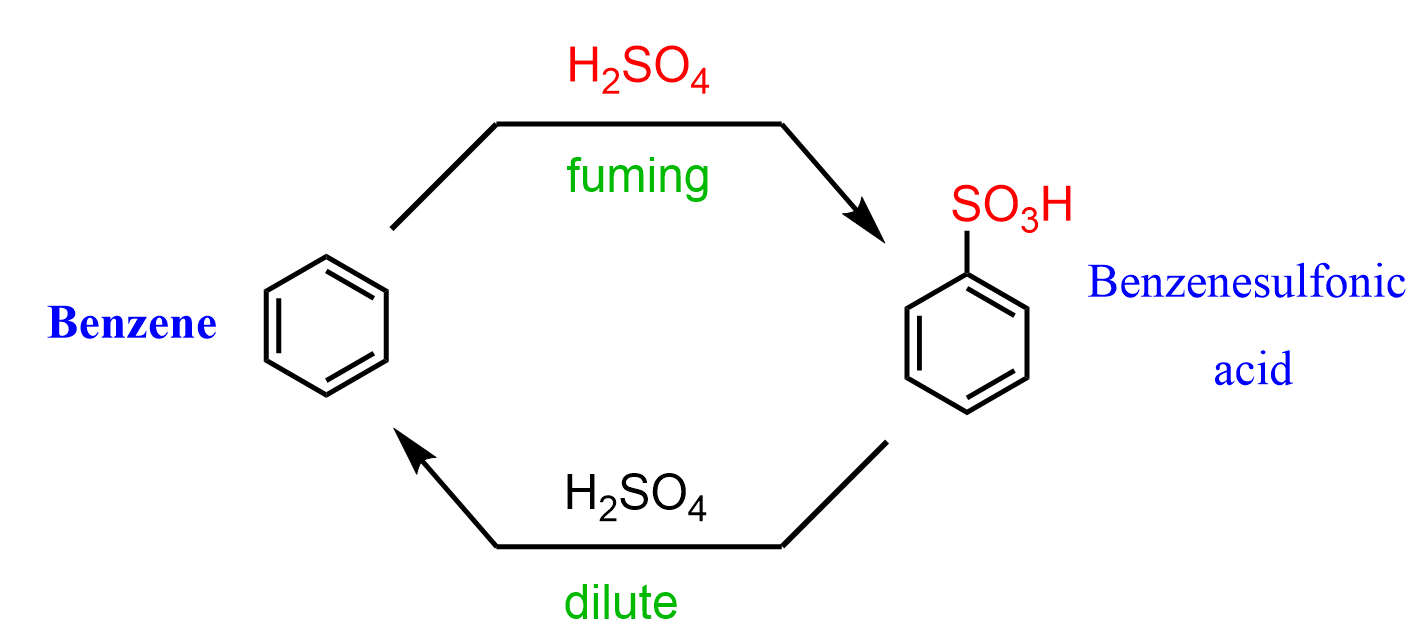
The sulfonation of benzene is typically achieved by reacting with fuming sulfuric acid, which is a mixture of H2SO4 and sulfur trioxide (SO3) known as oleum. The reaction follows an electrophilic aromatic substitution mechanism, producing benzenesulfonic acid.

The term “fuming” is given because of the reaction between SO3 and moisture in the air, producing an aerosol of concentrated sulfuric acid. The electrophile in this reaction is believed to be the protonated sulfur trioxide (+SO3H) or the SO3 by itself. The cationic intermediate (+SO3H) can be formed either by a reaction between SO3 and H2SO4 or protonation of sulfuric acid with another:
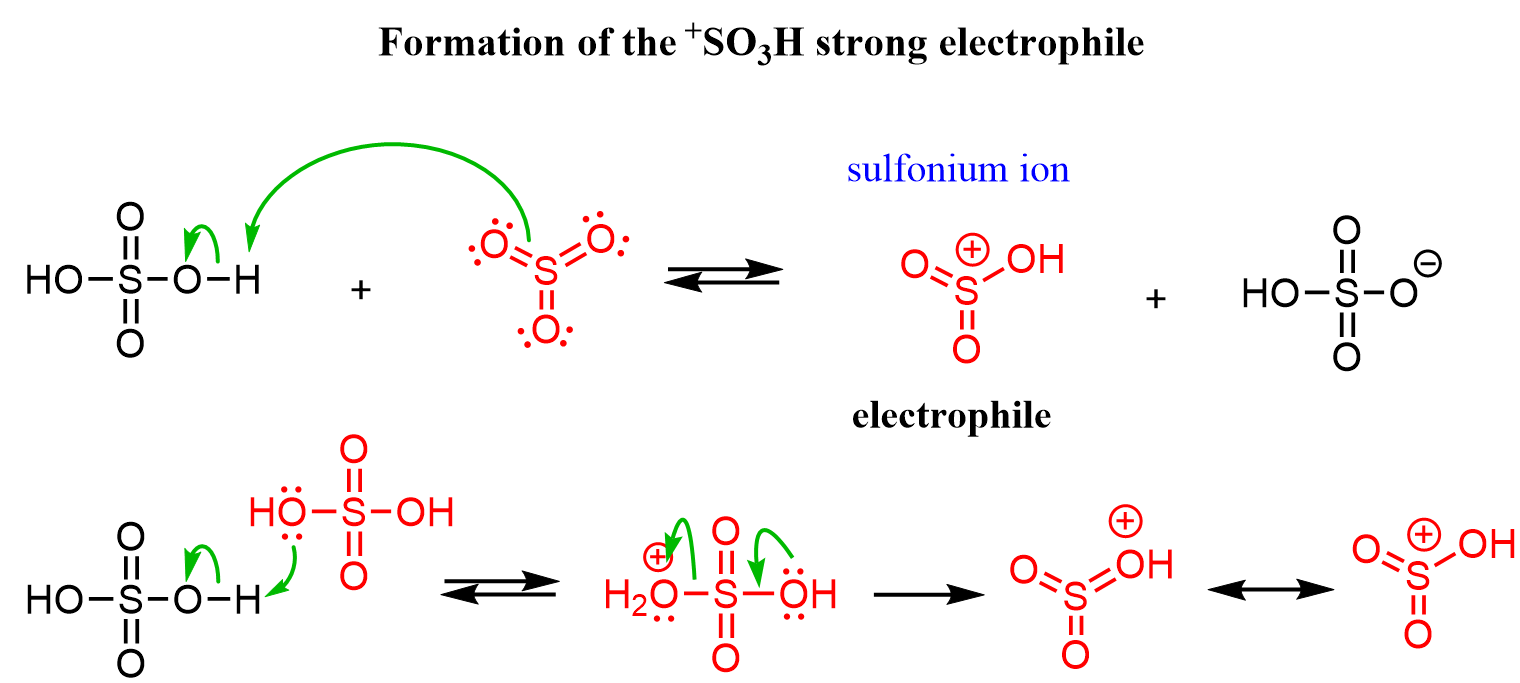
Once the electrophile is formed, it is attacked by the electrons of the aromatic system according to a standard electrophilic aromatic substitution mechanism.

The mechanism is shown interchangeably with the +SO3H or the SO3, and it is worth mentioning that sulfur trioxide itself is very electrophilic because of the three oxygen atoms pulling the electron density from the sulfur, so we can show it as the electrophile as well:

Desulfonation Reaction
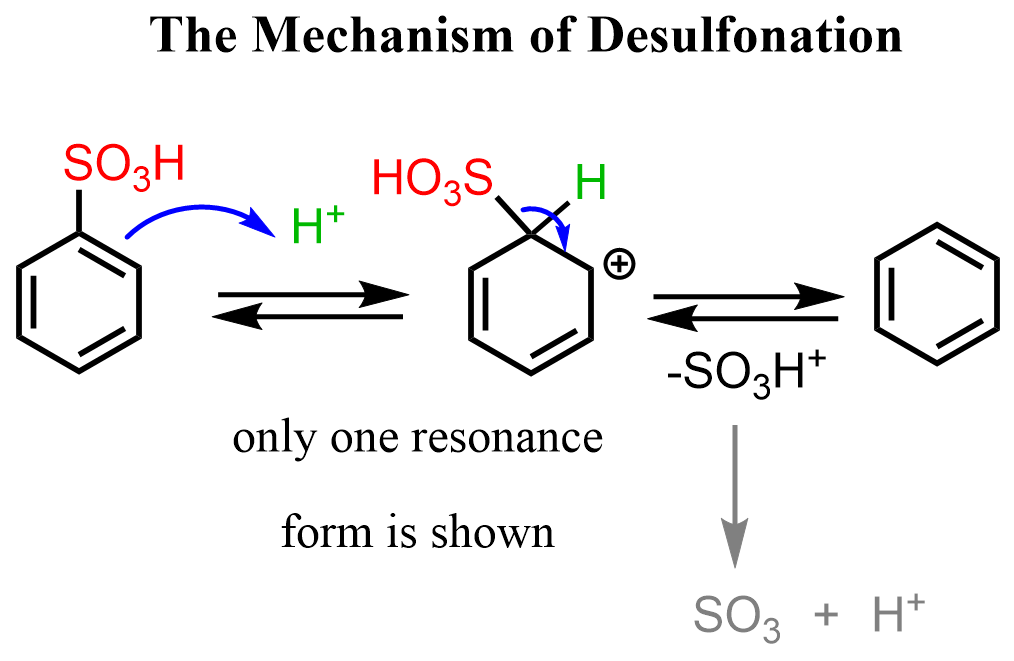
The sulfonation of benzene and derivatives is a reversible reaction. If a solution of dilute sulfuric acid is used, benzenesulfonic acid is desulfonated – the sulfonic acid group comes off the ring:
The mechanism of desulfonation is similar to the sulfonation, as it is another EAS reaction. In the first step, the ring is protonated and after the formation of the sigma complex, the +SO3H group is expelled from to ring, restoring the aromaticity:
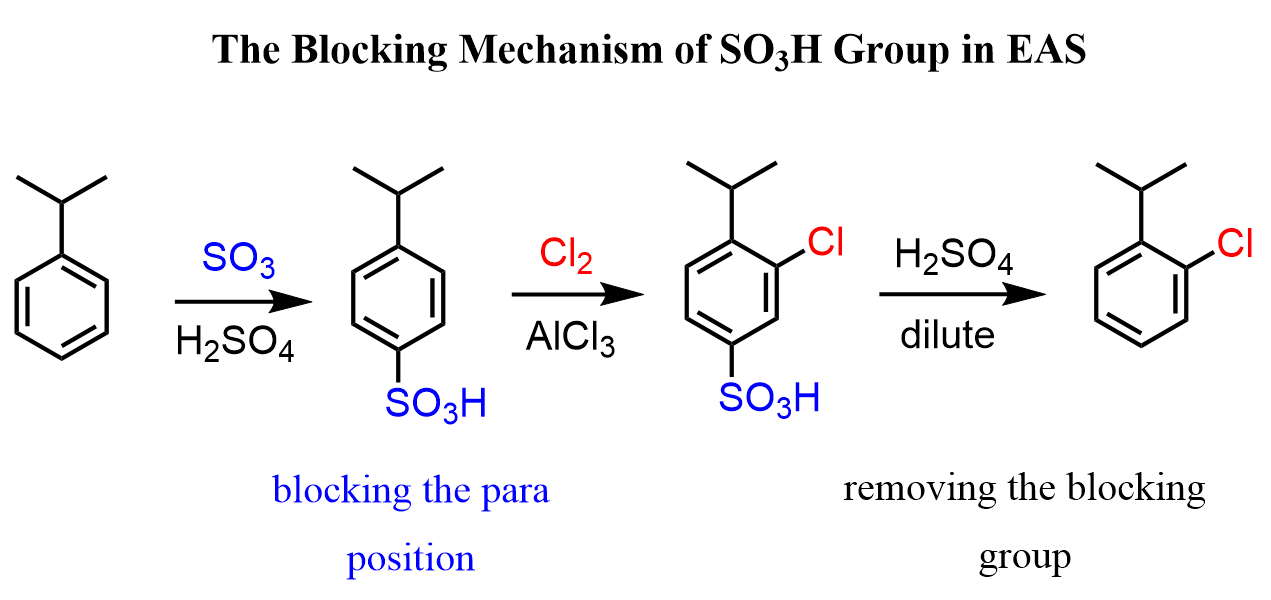
This is a very applicable feature of the sulfonate group as it can be used as a temporary blocking mechanism for synthesizing a specific regioisomer by EAS. For example, the chlorination of isopropylbenzene (cumene) gives the following isomer (para) as the major product because of the steric hindrance of the ortho (adjacent) position caused by the isopropyl group :
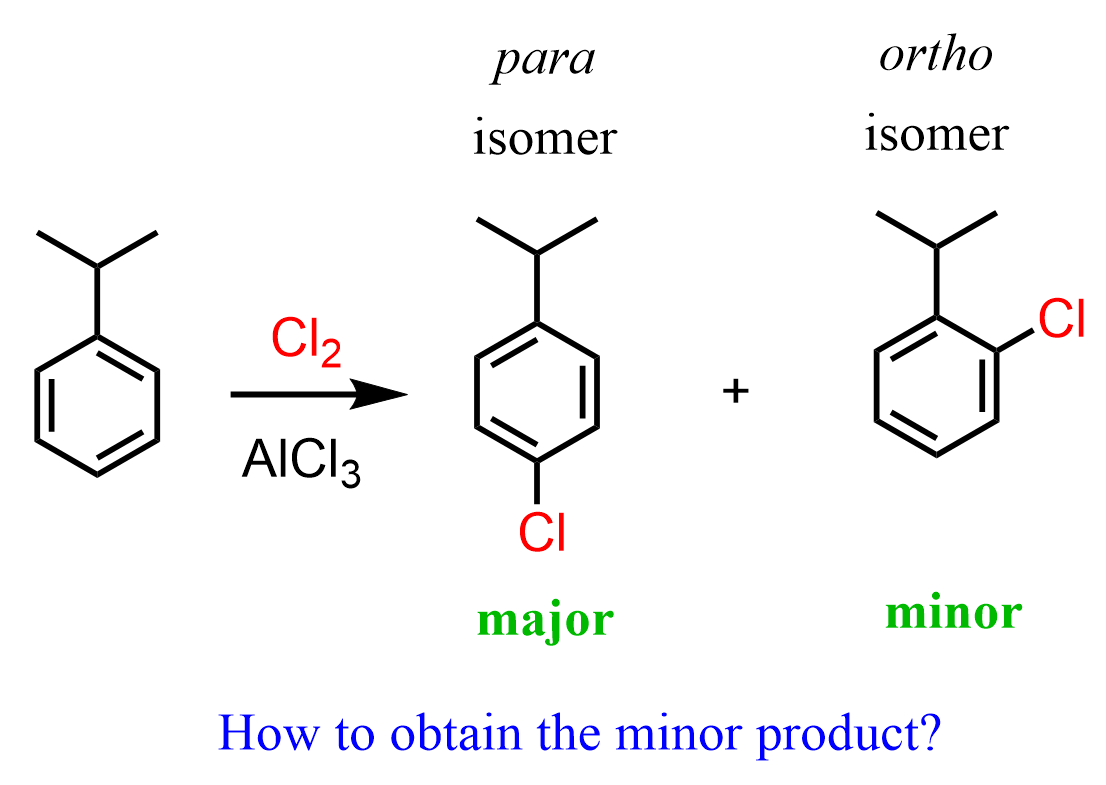
If we wanted the minor product, we could install the SO3H group, blocking the para position, and then brominate this intermediate at the ortho position, and finally remove the blocking group to obtain the desired ortho regioisomer:
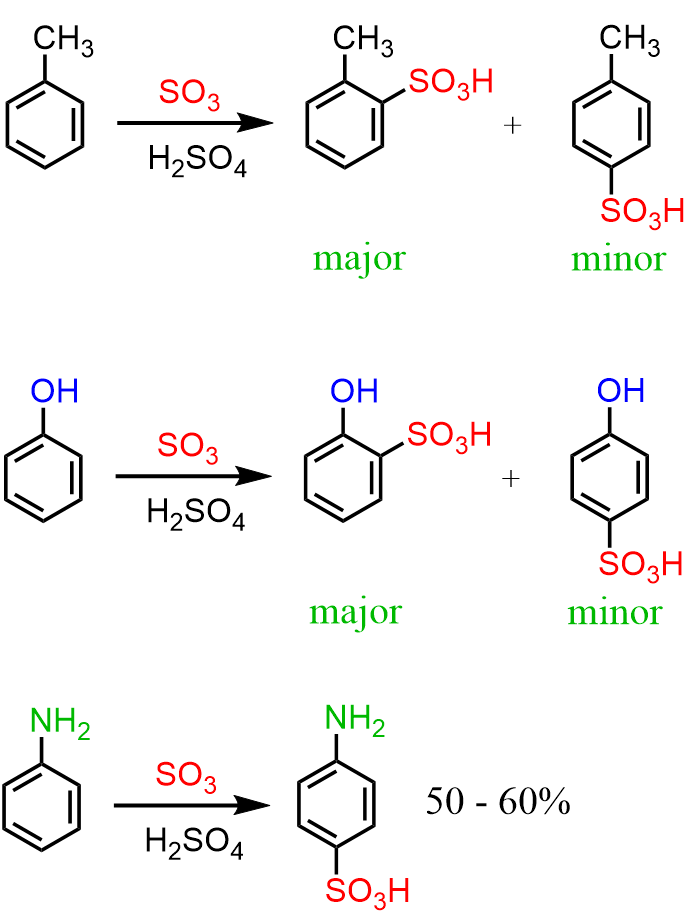
Sulfonation can be performed with many benzene derivatives, such as toluene, anisole, phenol, and aniline:
Let’s briefly discuss the isomers formed in electrophilic aromatic substitution, but there is a separate post about ortho, para, and meta substitution which you can find here.
The Effect of Sulfonation on the Reactivity of Benzene
You may not have covered this yet, but all the groups connected to the benzene ring are classified as activators and deactivators. Activators are those that make electrophilic substitution reactions (EAS) faster/easier compared to benzene, and deactivators are those that slow down or make certain EAS reactions impossible. Most activating groups are ortho, para, and deactivating groups are meta directors:

In the examples above, we can see that the methyl, OH, and NH2 groups are ortho, para directors. The ratio of the ortho and para substitution is difficult to predict. On one hand, the para position is favored because of the steric hindrance of the ortho position; on the other hand, there are two ortho positions and only one para, which statistically increases the yield of the ortho substitution. The larger the group present on the ring, the more favored the para substitution would be.
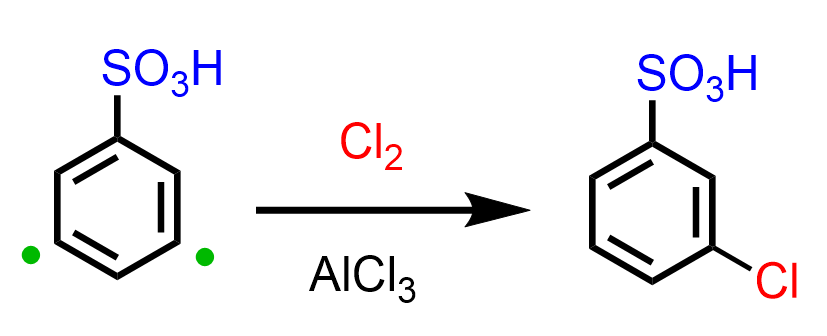
The sulfonate group, on the other hand, is a moderate/strong deactivator and hence a meta director. For example, the halogenation of benzenesulfonic acid produces a meta-halogenated derivative of the acid:
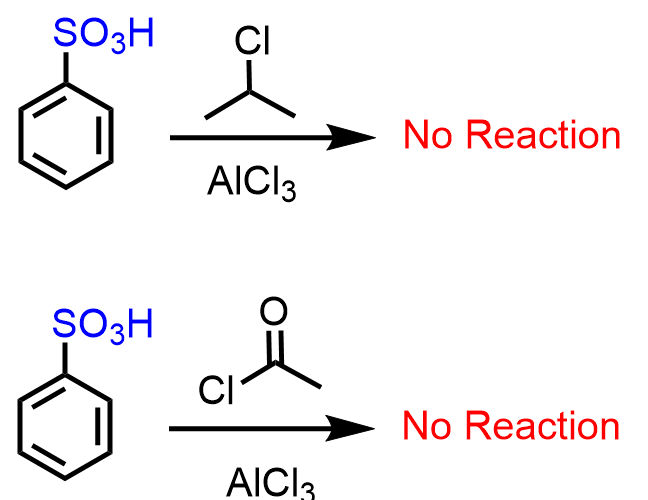
Being a strong deactivator, the SO3H makes some slow EAS reactions, including Friedel-Crafts alkylation and acylation, impossible:
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para– Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds

Benzene undergoes sulphonation and forms benzenesulphonic acid. Methylbenzene also undergoes a similar electrophilic substitution reaction of sulphonation as benzene. So what are the two products formed since we already are aware that methylbenzene produces two products, it bearing a methyl group which is an ortho-para director. So what are the two products formed in this case? The Ortho and the Para products.
The two isomers formed in the sulfonation of toluene are ortho-toluenesulfonic acid and para-toluenesulfonic acid (PTSA, pTSA, or pTsOH).
The para isomer is usually the major product, since steric hindrance between the methyl group and the bulky sulfonic acid group makes substitution at the ortho position less favorable.
The names 2-Methylbenzenesulfonic acid (o-methylbenzenesulfonic acid) and 4-Methylbenzenesulfonic acid (p-methylbenzenesulfonic acid) can also be used for naming these molecules.