In the previous post, we learned about ortho/para and meta-directing effects of various groups and atoms. Except for halogens, ortho/para directors are those who activate the benzene ring in electrophilic aromatic substitution, whereas deactivators direct the substitution in the meta position. Here is the summary of the directing effect for the most common groups and atoms you’d see in EAS reactions.

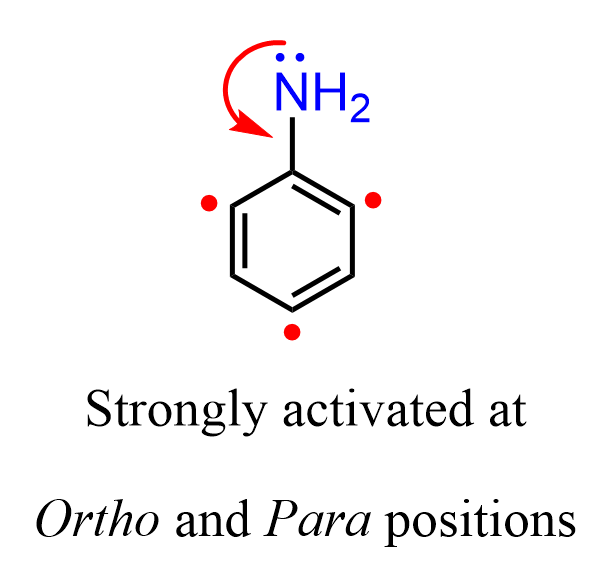
Recall that the directing effect occurs via inductive and resonance effects, and the resonance effect generally predominates. For example, the nitrogen in aniline deactivates the ring via an inductive effect because it is more electronegative than carbon; however, overall, it is a strongly activating group because of the resonance-donation by the lone pairs on the nitrogen. As a result, aniline undergoes electrophilic aromatic substitutions at the ortho and meta positions, often without the need for a Lewis acid catalyst:
The Effect of Phenyl Group on EAS Reactions
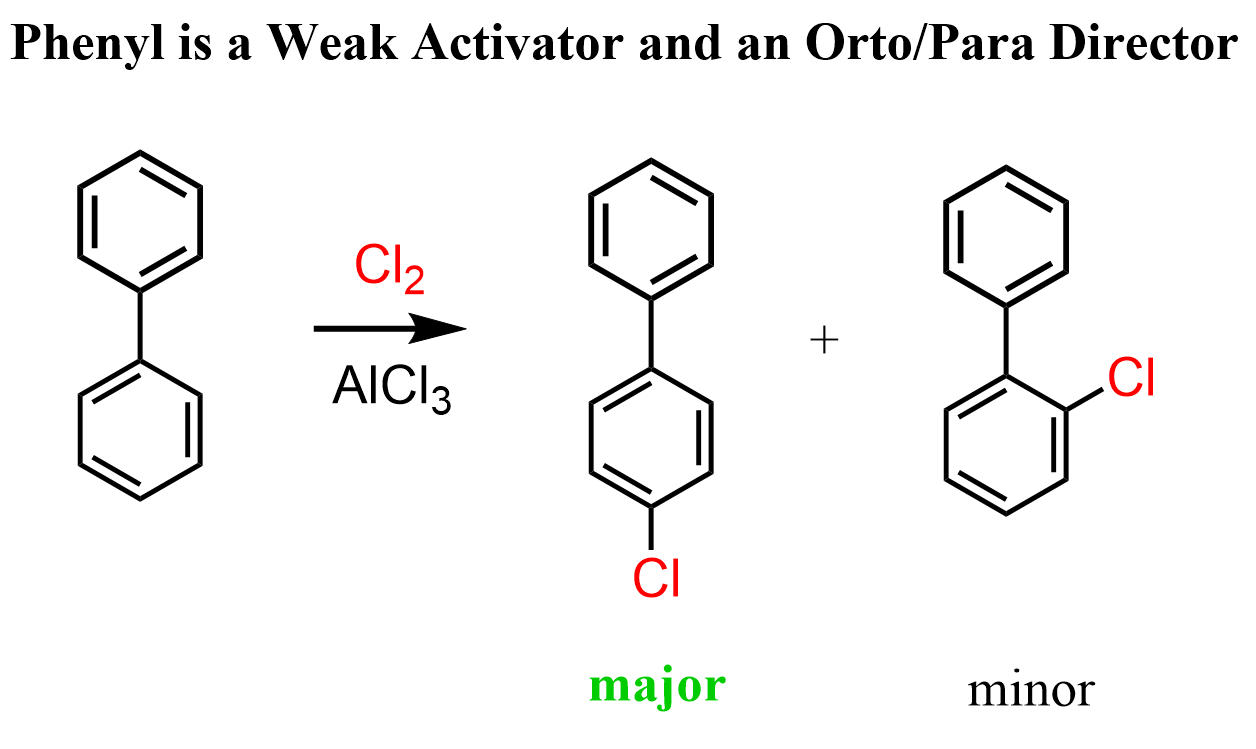
The phenyl group is a weak activator and thus an ortho/para director in EAS reactions. For example, the chlorination of biphenyl occurs mainly at the 4’ position which is the para position relative to the other ring:
As we have seen in many examples of EAS reactions, the substitution at the para position is favored because it allows for the delocalization of the positive charge over the two aromatic rings.
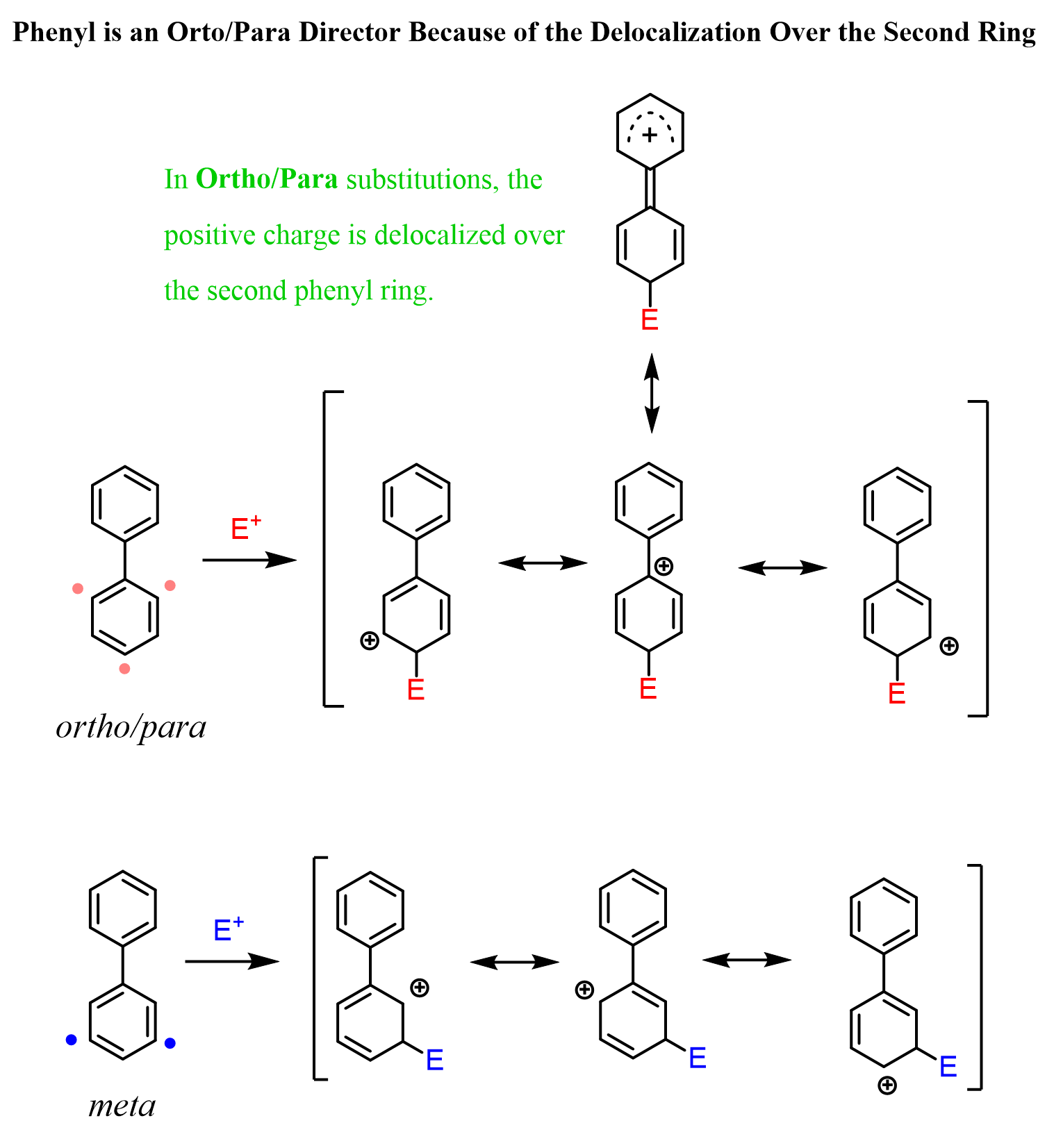
The ortho position is also favored by resonance-stabilization, however, notice that it is sterically hindered, and larger electrophiles produce less of the ortho-substituted isomer. For example, the Friedel-Crafts acylation of biphenyl almost exclusively gives the para product.

Reactions of Substituted Biphenyl
Let’s consider the bromination of biphenyl – specifically where does the second bromination occur?
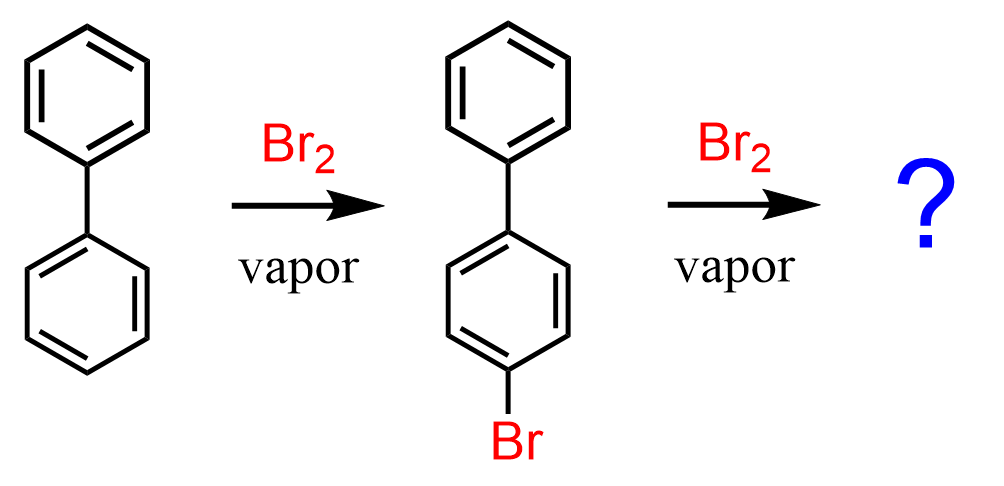
It has been shown that the second bromination occurs at the para position of the unsubstituted ring Org. Synth. 1951, 31, 29, DOI: 10.15227/orgsyn.031.0029:
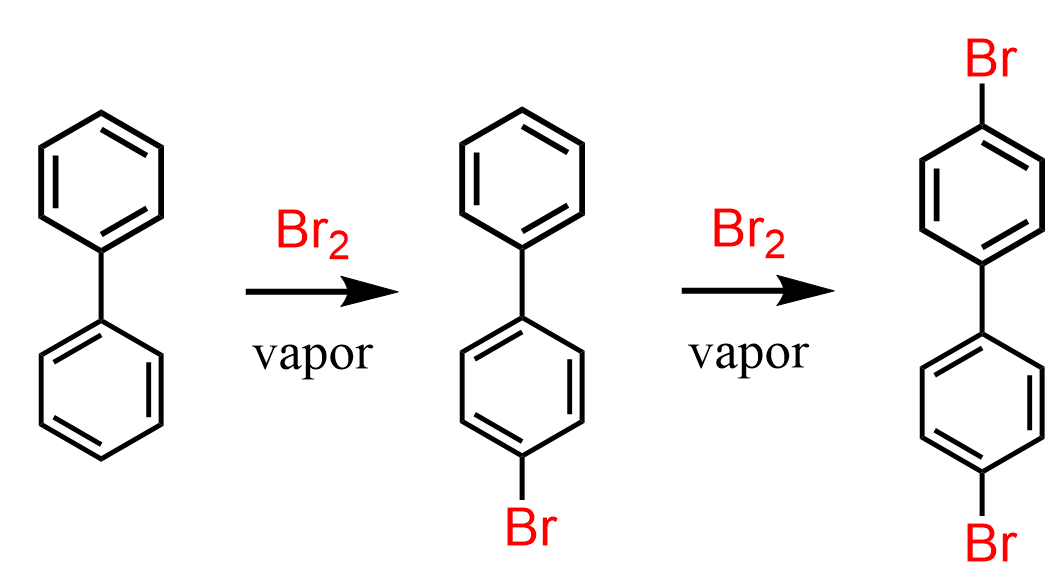
This is somewhat expected as we know that halogens, although deactivators, they are still ortho/para directors. So, the question really was would the bromine go the substituted or the unsubstituted ring:
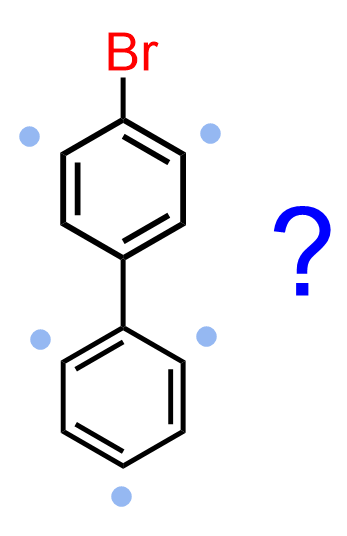
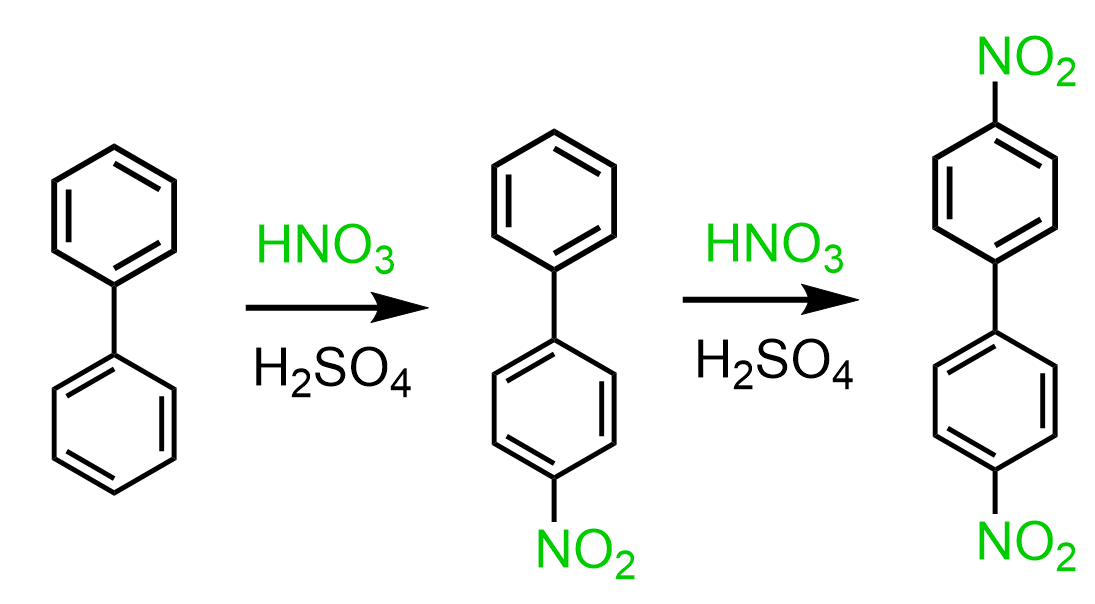
An interesting feature of substituted biphenyl is that irrespective of the electronic nature of the current group, the electrophilic substitution of the second group occurs in the 4’ position of the second ring (from RSC-Tutorial Chemistry Texts). For example, the nitration of 4-Nitrobiphenyl gives 4,4′-dinitro-1,1′-biphenyl even though the NO2 is a deactivator and a meta director.
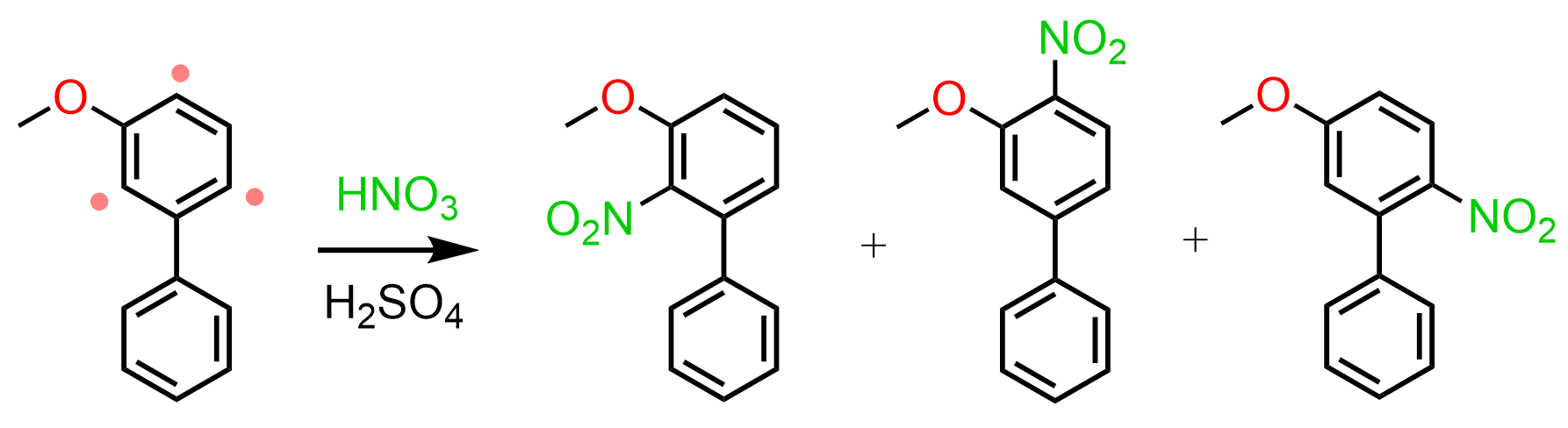
Let’s also consider a reaction of a meta-substituted biphenyl:
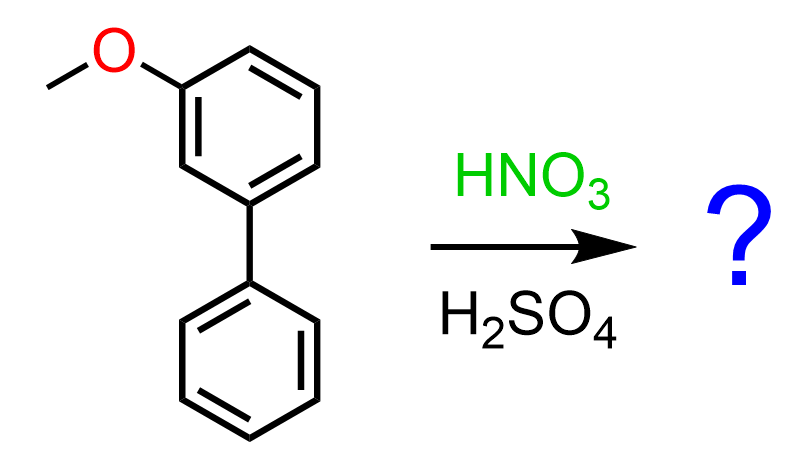
Unlike para-substituted biphenyls, the activating methoxy group can only activate the first ring via resonance donation, therefore, the EAS occurs on this ring (Loudon – Organic Chemistry):
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho Para Meta in EAS with Practice Problems
- Ortho Para and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators ?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
