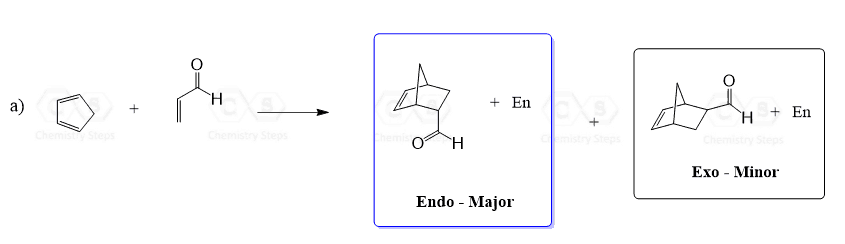
When a cyclic diene is used in the Diels-Alder reaction, a bridged bicyclic compound is formed:
This looks ordinary until we draw the product from a side view, which reveals some nice structures and interesting features of the mechanism that lead to the formation of two stereoisomers.
The diene and the dienophile can have two alignments. In one of them, the electron-withdrawing groups of the dienophile are pointing towards/underneath the diene, and in the second one, these groups are pointing away from the diene. These two orientations lead to the formation of two products – endo and exo:

In the endo product, the substituents of the dienophile are pointing towards the larger bridge, while in the exo isomer, they are pointing away from the larger bridge:

In general, endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. This decreases the energy of the transition state because of a favorable interaction between the non-bonding orbitals of the diene and the dienophile:

The exo product, on the other hand, is more stable as the substituent of the dienophile is pointing away from the larger ring system. Therefore, it is the thermodynamic product (more stable) and the endo is the kinetic product (forms faster).
*Note: The orbitals are shown in a simple way without indicating the phases. This is more complicated than it looks, and there are challenges in favor of and against any arguments.
Relationship of products in the Diels-Alder reaction
Let’s also address the relationship of the exo and endo products. In our example, both products are meso compounds as they have stereogenic centers, but the symmetry plane makes them achiral. The atoms are connected in the same way, yet the molecules are not mirror images of each other, and since they contain a plane of symmetry, they are achiral diastereomers.
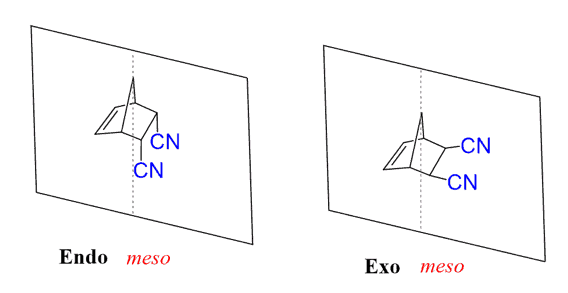
Check the post “Cis and Trans Isomers” to review the principles of this type of isomerism in cyclic structures, and to see why such compounds are considered diastereomers.
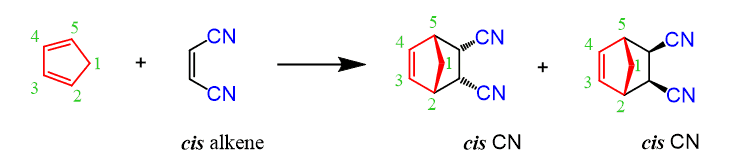
If the dienophile is unsymmetrical, then the product contains one stereogenic center, and it is now chiral since there is no plane of symmetry. The endo and exo products are formed as two enantiomers depending on the alignment of the diene and dienophile:

And any combination of an endo and exo product represents a pair of diastereomers:

If the diene is also unsymmetrical, then you need to consider the regiochemistry of the Diels-Alder as well. It would be too much for today, so you can check this post.
Endo and Exo for Acyclic Dienes
Sometimes the endo-exo definition is broadened to include acyclic dienes as well. Here is what you need to know if your instructor requires this. First, remember that the groups on a substituted diene are classified as inner and outer, and these pairs always appear on the same side (cis) in the product of any Diels-Alder reaction:
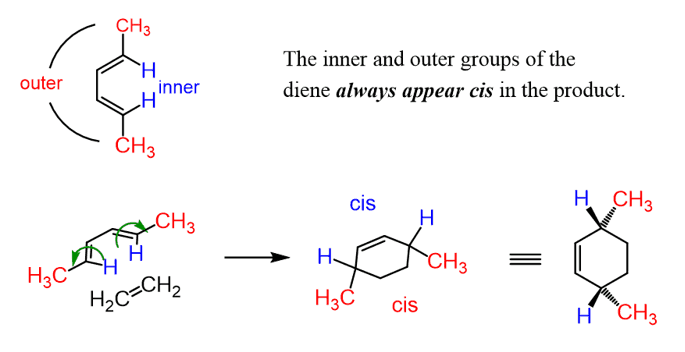
It is graduate level, and if you need more details, read the Woodward–Hoffmann rules for the pericyclic reactions according to which the 4+2 cycloadditions occur via disrotatory movement (indicated by green arrows above) of the frontier molecular orbitals.
Back to our reactions. What would the stereochemistry be if the alkene were substituted too?
Here is a good trick to understand the stereochemistry:
Imagine that the two inner hydrogens are connected and draw the product as we did for a cyclic diene – hydrogens pointing up, methyl groups pointing down. You can also do this by adding an imaginary carbon to temporarily turn it into a cyclic diene:

Notice that in the endo product, the substituents on the diene and dienophile are cis as they are both pointing in the same direction. And this is the broader definition of the endo product pertaining to cyclic and acyclic dienes.
Summary of Endo and Exo Products
The stereochemical outcome of Diels–Alder reactions varies depending on the structure of the diene and the reaction conditions. Most often, you will hear or use the terms endo and exo, which, although universal, are more applicable to cyclic products.
-
Endo refers to the product where the substituents of the dienophile (usually electron-withdrawing groups) point toward the larger bridge of the bicyclic system.
-
Exo refers to the product where those substituents point away from the larger bridge.

By habit, we often refer to the endo product as the “major product,” but keep in mind that the endo product is the kinetic product that forms faster because of a favorable interaction between the π system of the diene and the electron-withdrawing substituents of the dienophile in the transition state (secondary orbital overlap).
The exo product, on the other hand, is generally the thermodynamic product because it places bulky substituents in a less hindered position, making the product more stable overall. To favor the formation of the thermodynamic product, the reaction is carried out at higher temperatures.