In this post, we’ll talk about a common question you encounter in organic chemistry tests and exams. How do you know if the alkyl halide or a similar substrate will react by SN1 or E1 mechanism?
This dilemma is one branch of a larger question tree where you need to determine if the reaction follows SN1, SN2, E1, or E2 mechanism. We have a separate discussion on this topic, so go ahead and check it out here.
In short, you will only need to choose between SN1 and E1 mechanisms when a weak base/nucleophile is used. The use of a strong base/good nucleophile indicates either an SN2 or E2 mechanism.

The most common weak bases you will encounter in elimination reactions of alkyl halides are water and alcohols. Once again, these are also poor nucleophiles and therefore, they only do SN1 and E1 reactions. Keep in mind also that the words “only”, “never”, and “always” are always associated with exceptions, so we are talking about the general patterns.
When is the SN1 Mechanism Foavored?
Let’s mention this one more time: You have a weak nucleophile such as water or an alcohol, so you know it is going to be SN1 or E1. In a typical organic chemistry 1 question, unless heat is mentioned, you need to go with the SN1 mechanism.
There are different types of SN1 reactions, such as those of alkyl halides with water or alcohols, those of alcohols when reacted with hydrohalic acids, HCl, HBr, or HI.
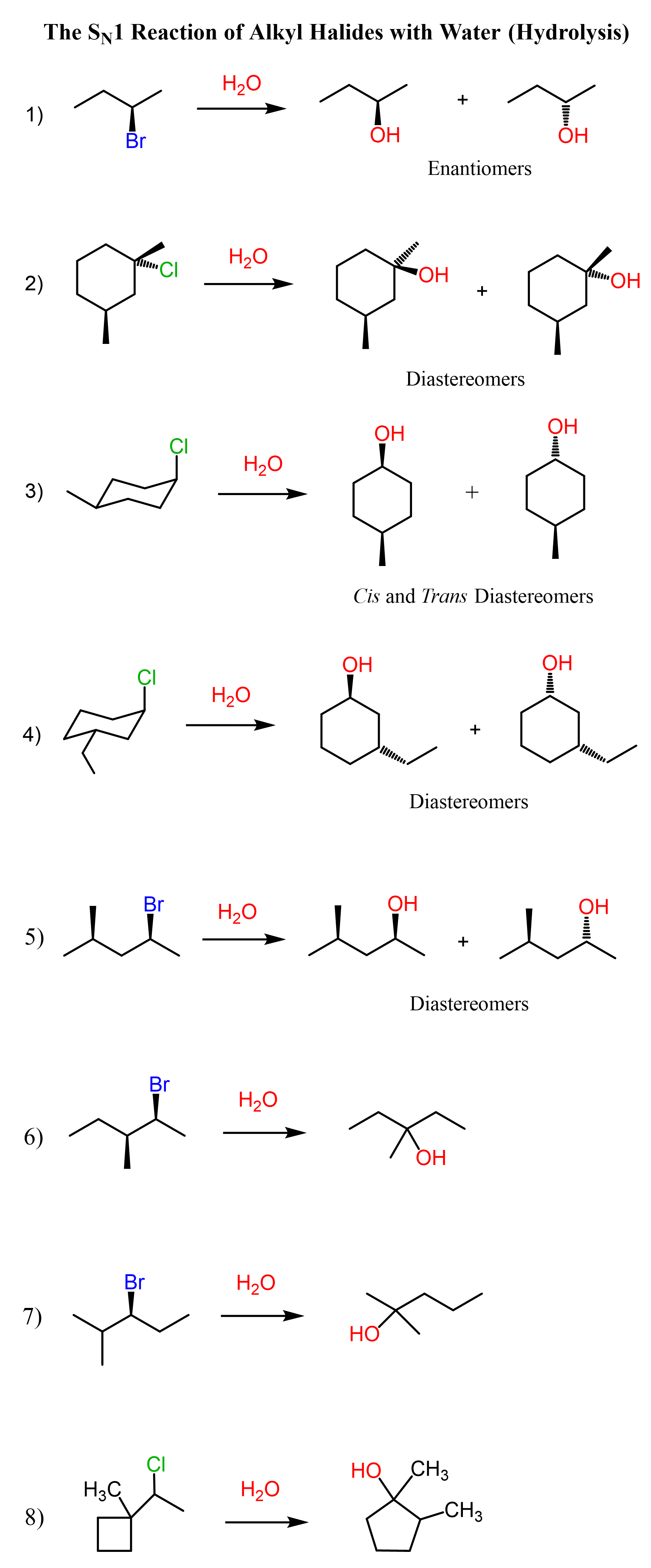
I will list a comprehensive collection of SN1 reactions between alkyl halides and water here, without delving into the mechanism and stereochemistry of each reaction, as this post focuses on deciding between SN1 and E1 reactions.
Check the linked article for more details, mechanisms, and practice problems in the SN1 reaction of alkyl halides with water.
What Factors Favor E1 over SN1?
As mentioned above, the competition between SN1 substitution and E1 elimination reactions is often determined by the temperature of the reaction. Recall that heat favors elimination, so the higher the temperature, the more elimination products we’d normally get. One reason for this is the formation of an extra molecule in elimination reactions, meaning that eliminations are entropically favored.
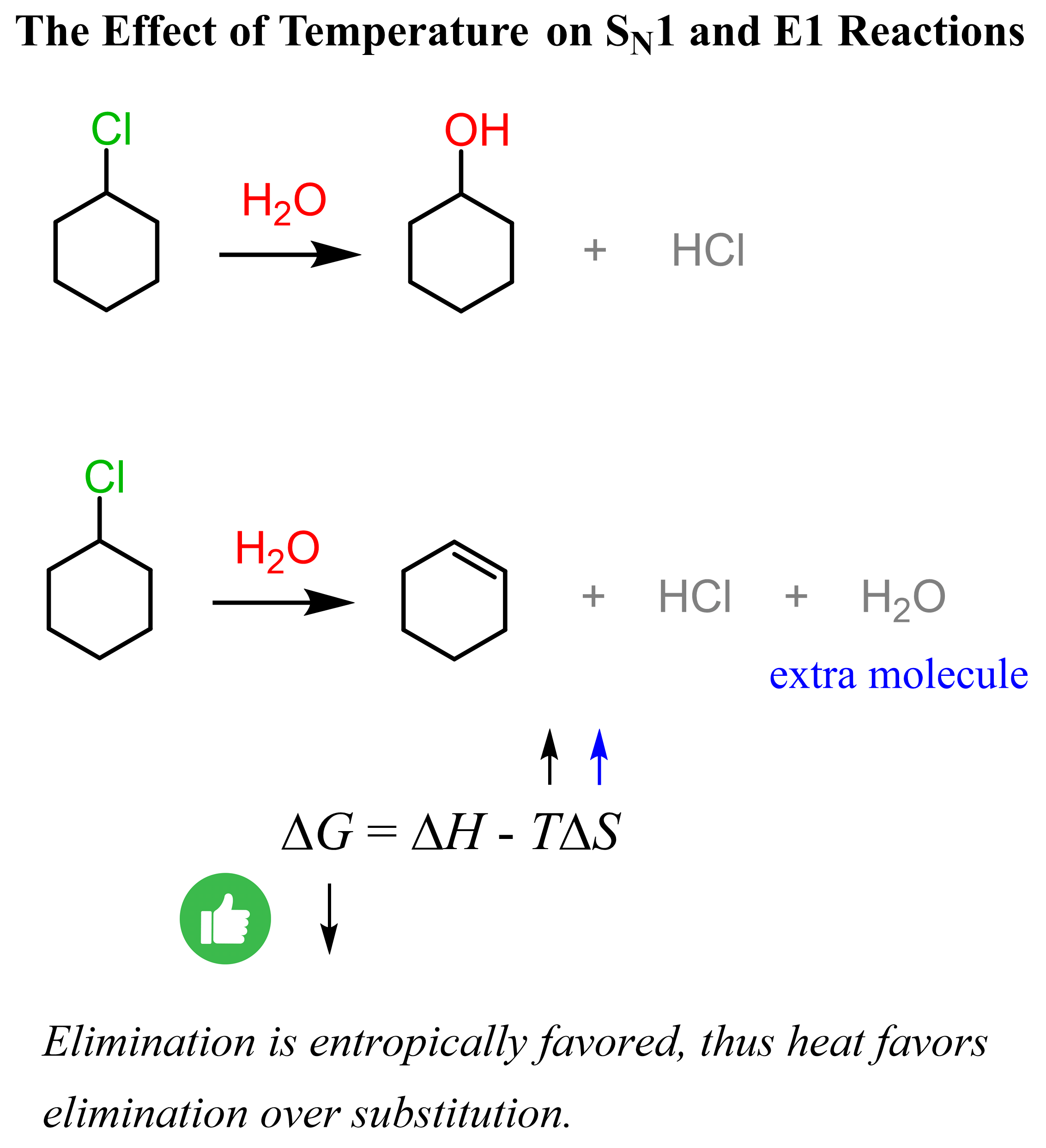
The extra molecule formed in the elimination reactions increases the entropy, and the higher temperature makes it a larger (negative) factor, which decreases the Gibbs free energy of the reaction. Recall that a negative ΔG indicates a spontaneous reaction, and in general, the smaller it is, the more favored the forward reaction. Check this article for more details about the effect of temperature on substitution and elimination reactions.
The Structure of the Product Alkene
Another factor that favors elimination is the formation of substituted and conjugated alkenes (dienes). Recall that the stability of alkenes increases with the number of alkyl groups on the double bond, and conjugated systems are also associated with additional stabilization.
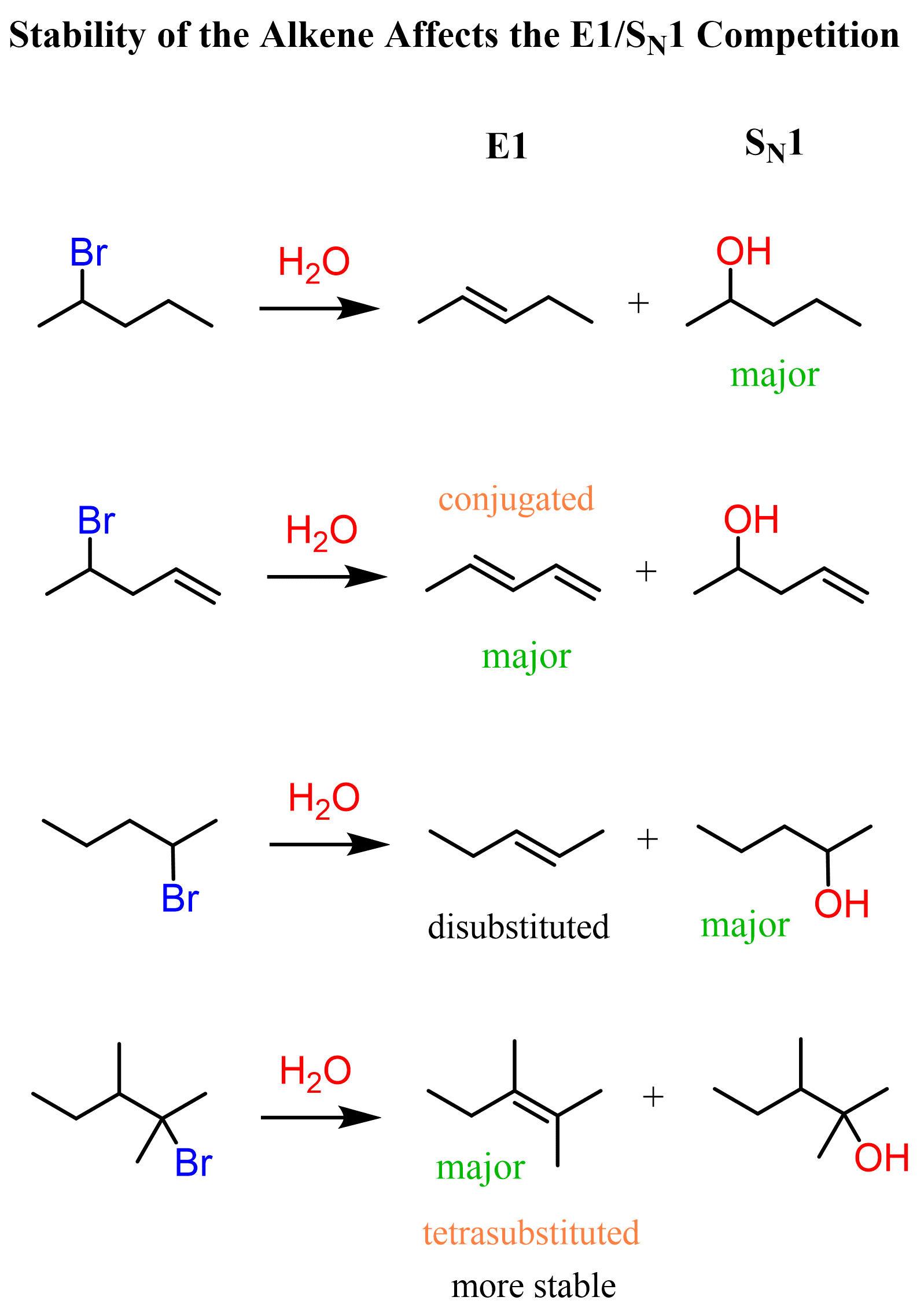
You can read about the stability of conjugated systems here, as well as in the article about aromatic compounds, which is an Organic 2 material, though.
I also want to mention that I did not search for experimental data to show the percentage of each product in these reactions. This is simply to explain what favors elimination and when we can expect it to be the major pathway of the reaction.
What is Common in SN1 and E1 Reactions?
Both SN1 and E1 reactions are unimolecular mechanisms that proceed via the formation of carbocations.
They only differ in that instead of a nucleophilic attack, the water now acts as a base, removing the β-hydrogen:

Very often, a mixture of products is formed, and there is no universal, clear-cut cut between the E1 and SN1 reactions. For example, the ratio of the alkene and alcohol formed in the reaction shown above will depend, for example, on the temperature.
This is also true for reactions that proceed via rearrangements. Remember, carbocations are prone to rearrangements whenever a more stable one can be formed. For example, the following secondary alkyl halide forms the corresponding carbocation in the solvolysis reaction, which then undergoes rearrangement, and a mixture of an alcohol and an alkene will be formed:

In Summary
- Both SN1 (substitution) and E1 (elimination) reactions occur under similar conditions when a weak nucleophile/base such as water or alcohol is used. These are often the solvent of the reaction, and therefore, we also call them solvolysis reactions. These reactions proceed through a carbocation intermediate, so they are most favorable with tertiary or resonance-stabilized substrates.
- When heat is applied, the reaction pathway tends to shift toward E1, since elimination is generally more entropically favored at higher temperatures. E1 is also more likely when the resulting alkene is more substituted (and thus more stable).
- The SN1 and E1 are competing reactions, and under typical conditions, you can expect to obtain a mixture of substitution and elimination products.
For more practice problems on SN1 reactions, check: The E1 Mechanism
For E1 elimination reactions: The E1 Elimination Mechanism and Practice Problems
For choosing between SN1, SN2, E1, and E2 reactions, refer to the set of practice problems here.
In addition to this, there is also a multiple-choice quiz on substitution and elimination reactions:
Nucleophilic Substitution and Elimination Practice Quiz
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
- Substitution and Elimination Reactions
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- SN2 and E2 Rates of Cyclohexanes
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism with Practice Problems
- Regioselectivity of E1 Reactions
- Stereoselectivity of E1 Reactions
- How to tell if it is E2 or E1 Mechanism
- SN2 vs E2 Reactions
- Dehydration of Alcohols by E1 and E2 Elimination
- Mesylates and Tosylates as Good Leaving Groups
- Mitsunobu Reaction
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Polar Protic and Polar Aprotic Solvents
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
- The Hammond Postulate
- The E1cB Elimination Mechanism
- Nucleophilic Substitution and Elimination Practice Quiz