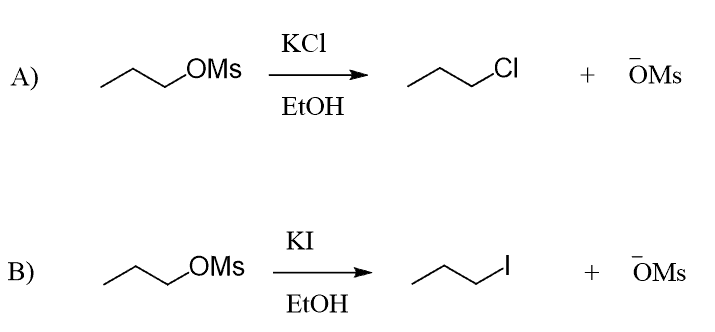
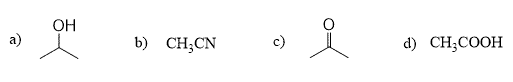You are here because you have almost figured out what polar protic and aprotic solvents are, but want to clearly understand what they have to do with SN1 and SN2 reactions, isn’t that right?
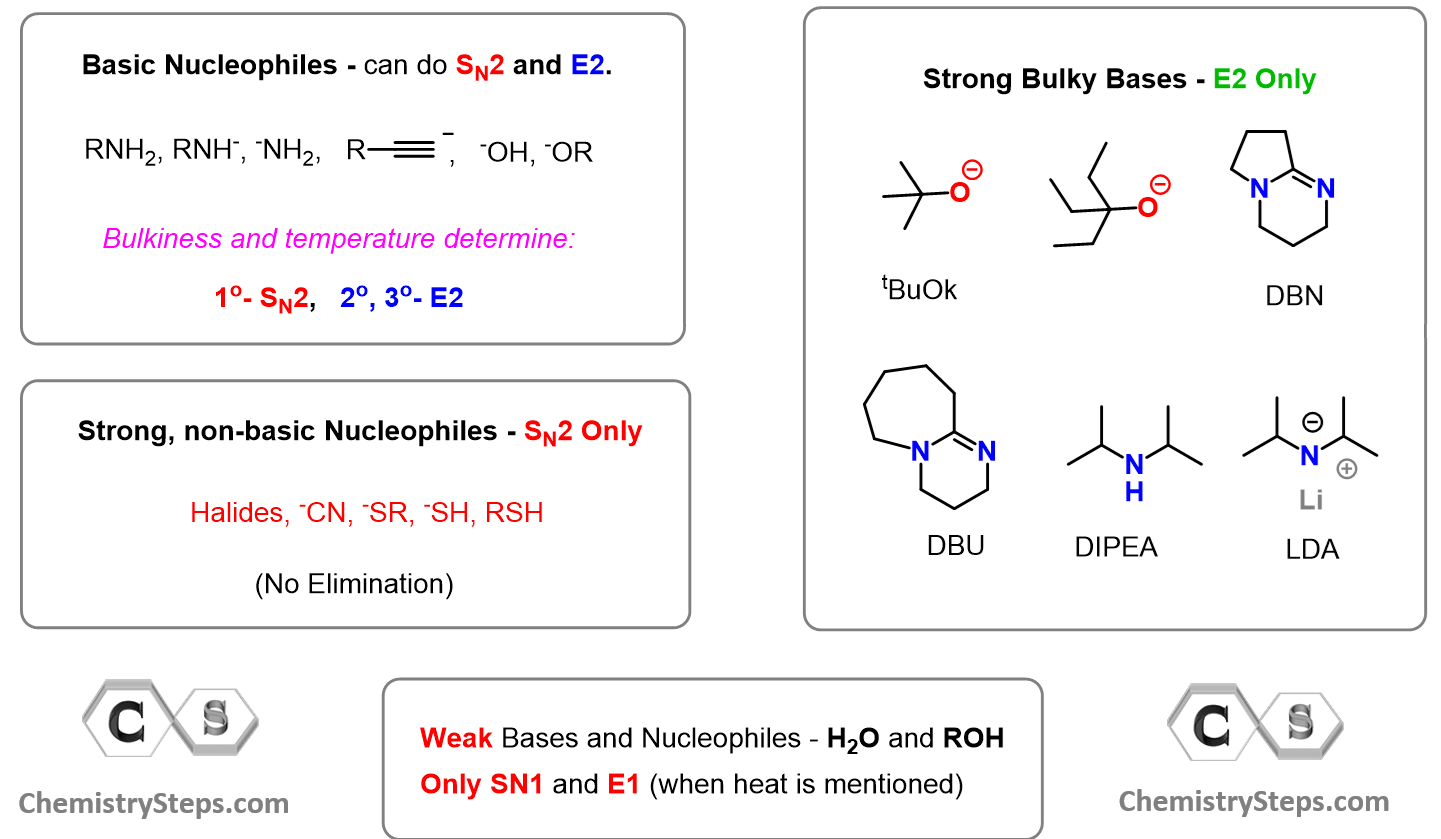
To answer and understand this, let’s first understand what makes a good nucleophile or a nucleophile in general. A nucleophile is a species, whether charged or not, that has some electrons available to attack an electron-deficient species (electrophile).
These are the most common nucleophiles and bases you will need to know in this chapter on Substitution and Elimination reactions. Notice that they are all electron-rich species:
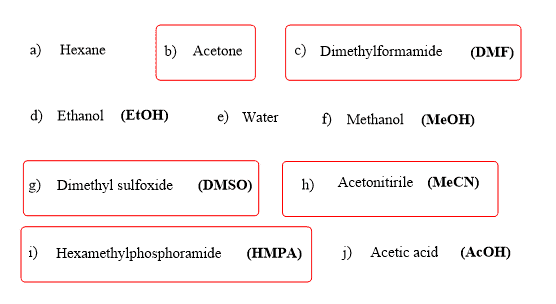
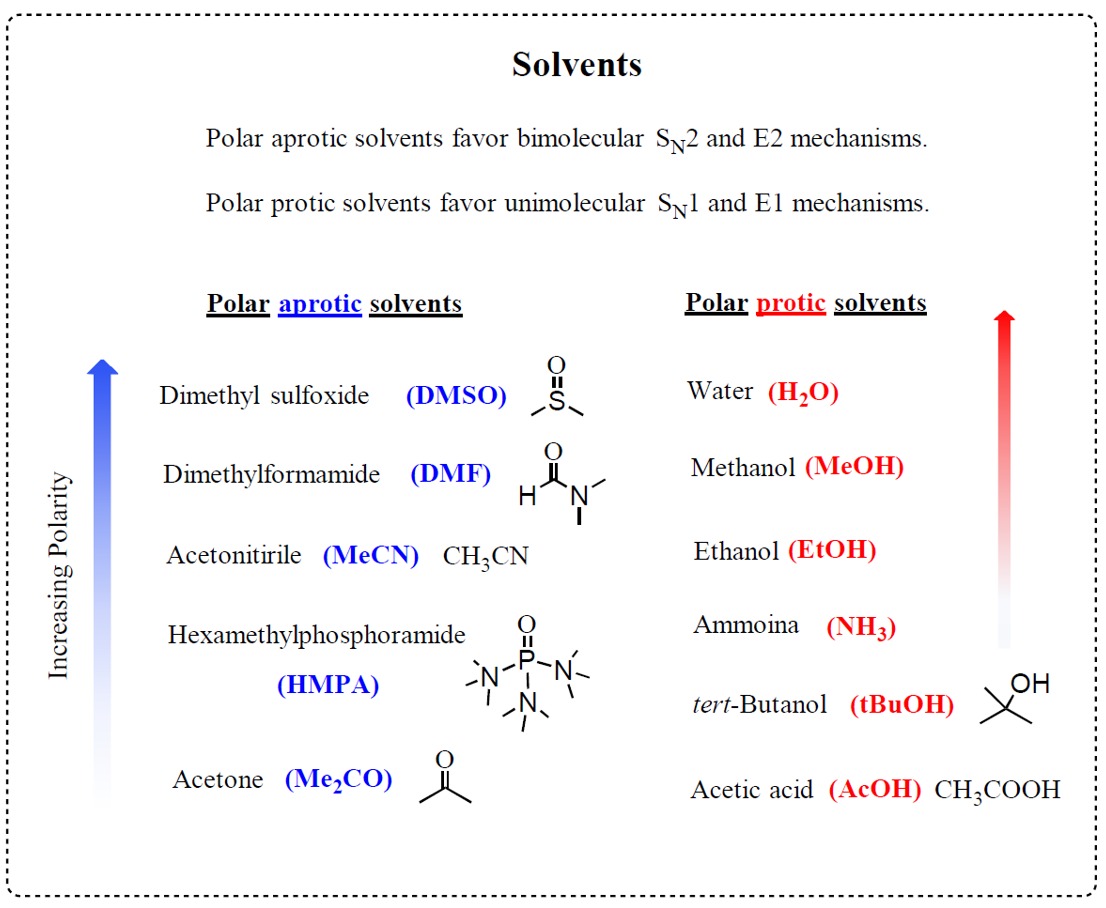
So, if we want to keep the nucleophile strong/good, we don’t want to distract these electrons. And the distraction is usually done by some solvents which are capable of interacting with the electron via hydrogen bonding. These are called polar protic solvents, such as water, alcohols, and acetic acid:
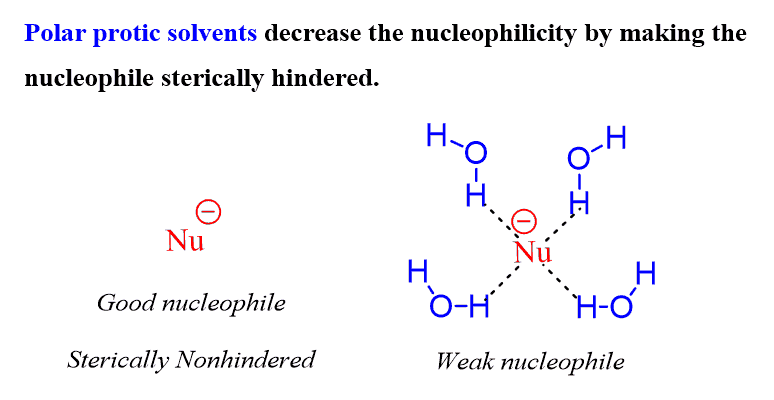
As a reminder, hydrogen bonding is an intermolecular interaction that occurs when a hydrogen atom covalently bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine is attracted to a lone pair of electrons on another atom. Hydrogen bonding decreases the nucleophilicity by distracting the electrons responsible for making the nucleophilic what it is.
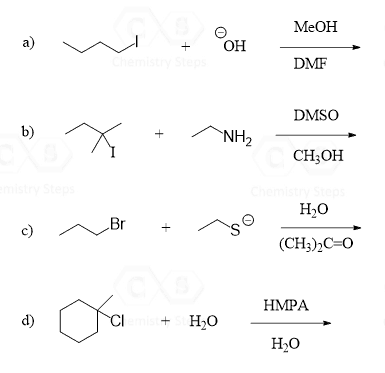
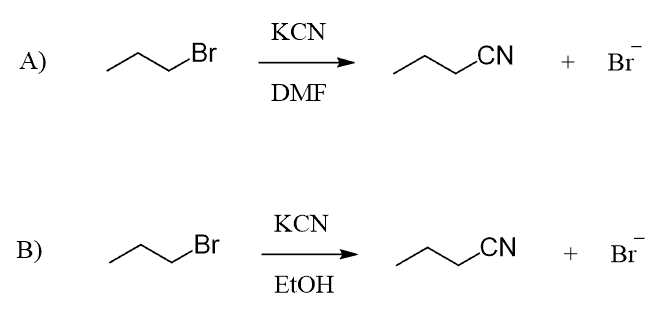
For example, we know that cyanide ion is a good nucleophile, and therefore, readily does SN2 substitutions on primary and secondary alkyl halides.
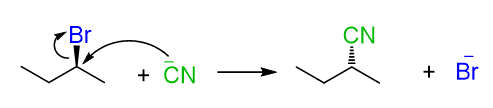
An important detail, often not shown in the equation, is the solvent used to facilitate the SN2 substitution. The solvent must lack hydrogen atoms connected to electronegative atoms to avoid the possibility of hydrogen bonding with the cyanide ion. In other words, it must be a polar aprotic (read it as non-protic) solvent. The most common polar aprotic solvents are DMF, DMSO, acetone, acetonitrile, etc.
When the reaction is run in a polar aprotic solvent, MeCN, the rate increases 5000 times compared to when methanol, a polar protic solvent, is used:
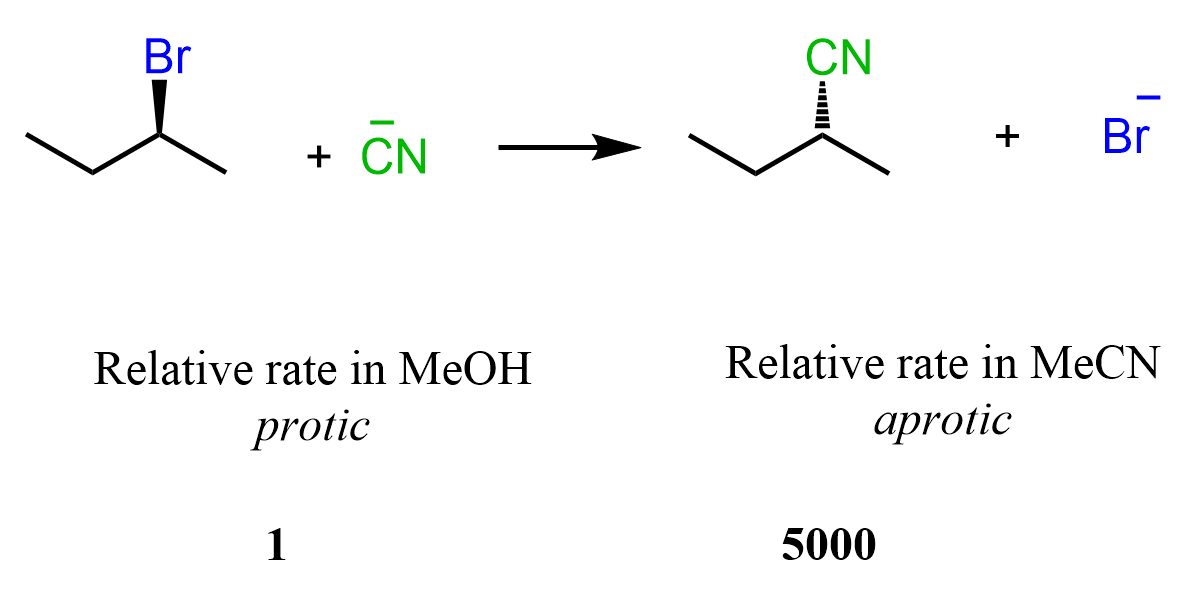
Once again, methanol has such a detrimental effect on the rate of this SN2 substitution because it cages the –CN nucleophile by hydrogen bonding with it. On the other hand, acetonitrile, MeCN, is a polar aprotic solvent great for promoting the SN2 mechanism as it is lacking a hydrogen capable of interacting, thus suppressing the nucleophilicity of the cyanide ion.
Here is the list of the most common polar protic and aprotic solvents you will see in your organic chemistry class:
To summarize the effect of polar protic and aprotic solvents, we can say that polar protic solvents cage both the nucleophile and the counterion. Therefore, using, for example, NaCl, alkoxide ions such as EtONa in alcohols, water, or other protic solvents makes them ineffective in SN2 reactions:

Polar aprotic solvents, on the other hand, are the ones without a hydrogen connected to an electronegative atom, and the key difference compared to polar protic solvents is the lack of intermolecular hydrogen bonding.
For example, when the salt is added to a solution of DMSO, only the sodium ions are solvated, and the Cl– stays now as a naked ion:

The Role of Solvent in SN1 Reactions
So far, we have talked about how beneficial polar aprotic solvents are for SN2 reactions, and how polar protic solvents suppress the nucleophilicity, thus the rate of SN2 reactions.
Now, what about SN1 reactions? Which solvents are best for this mechanism?
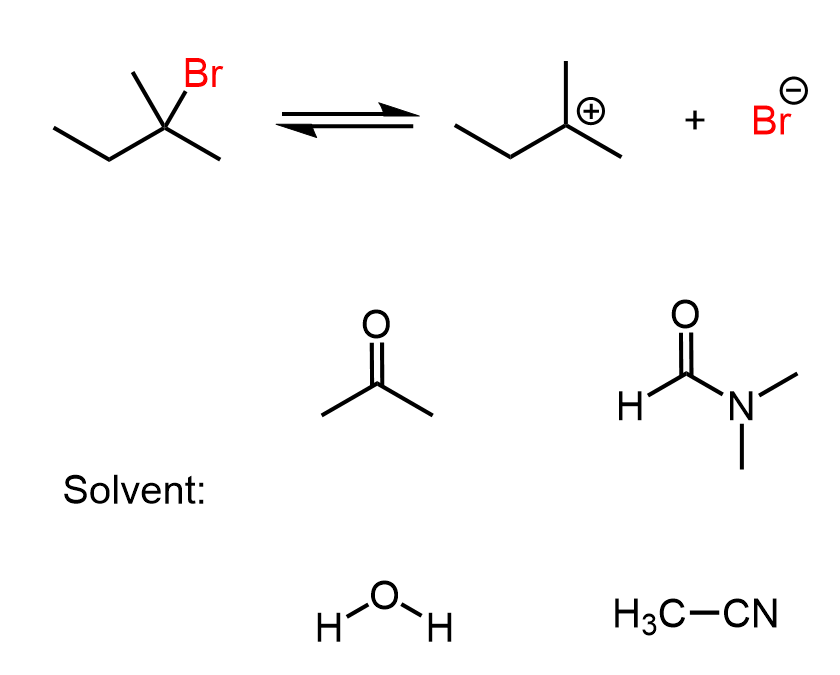
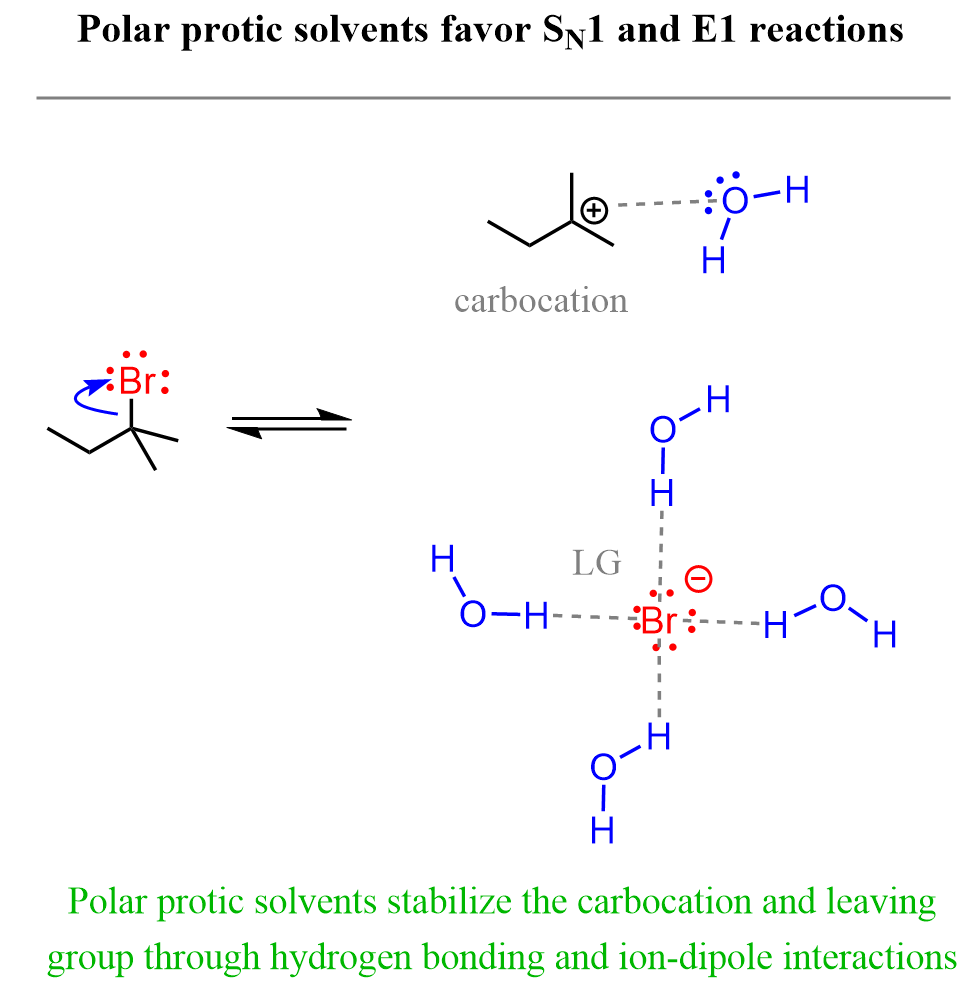
The first thing here is to recall that SN1 is a stepwise mechanism, and importantly, the rate-determining step is the loss of the leaving group, forming a carbocation intermediate. Therefore, if the solvent has any effect here, it must be by affecting the efficiency of the C–X bond breaking. For example, to make it faster, the solvent must stabilize the resulting carbocation and the halide ion (the leaving group).
What do you think, should a polar protic or aprotic solvent be used to stabilize these intermediates?
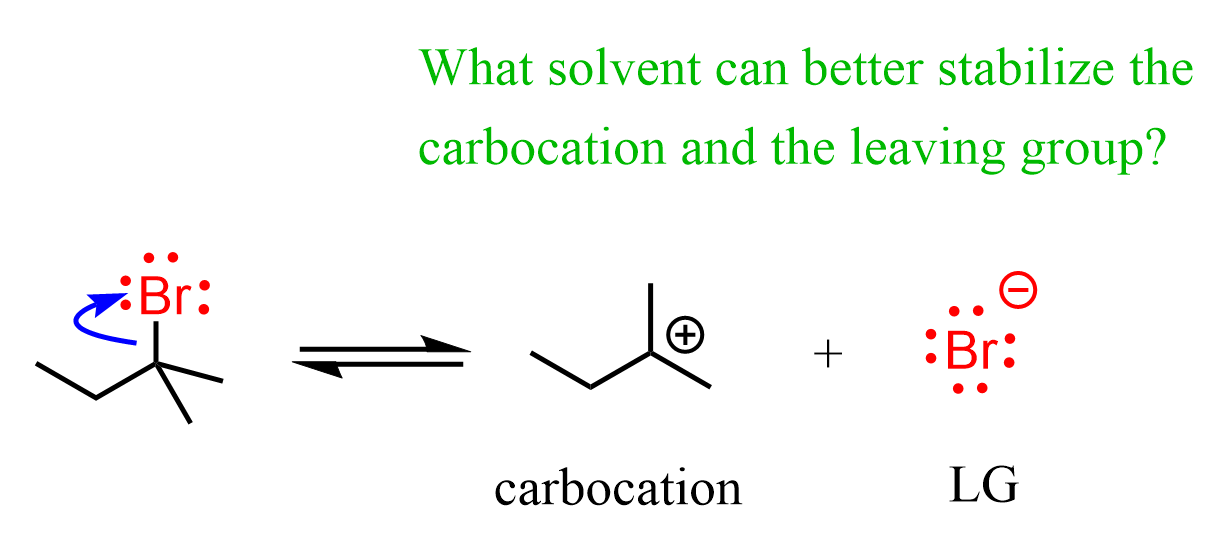
The carbocation is positively charged, and the leaving group is negatively charged. Polar protic solvents (e.g., water, methanol, ethanol, acetic acid) are a better choice for SN1 reactions, as they stabilize both the positively charged carbocation and the negatively charged leaving group through ion-dipole interactions and hydrogen bonding.
Like they did with the nucleophile, hydrogen bonds with the leaving group, thus stabilizing it and making the rate-determining step easier.
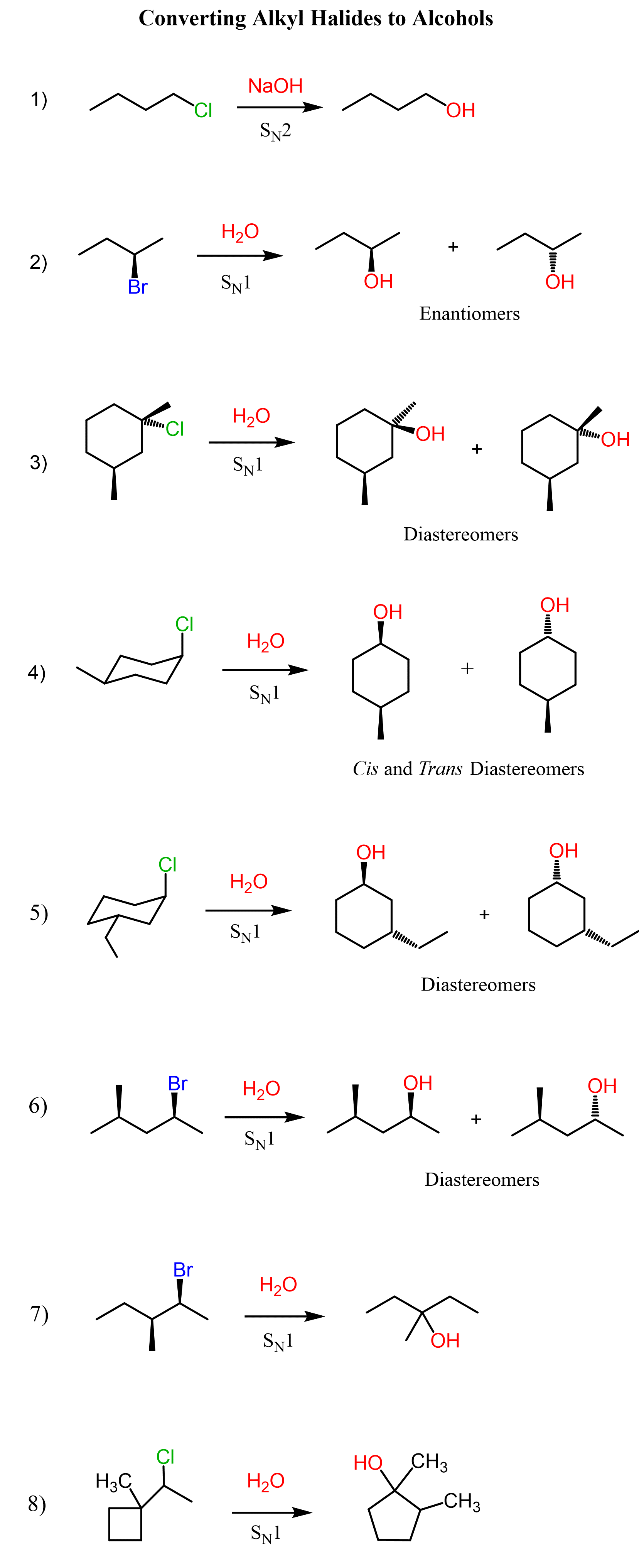
Here are some typical SN1 reactions where weak nucleophiles react with alkyl halides. Notice that water serves both as the nucleophile and the solvent, and these are known as solvolysis reactions.
Check these articles for more details and mechanisms of SN1 reactions and conversion of alkyl halides to alcohols.
To summarize this section, the most important pattern you need to remember for the effect of solvent in substitution reactions is that polar aprotic solvents favor SN2, and polar protic solvents favor SN1.
The Effect of Solvent in E1 Reactions
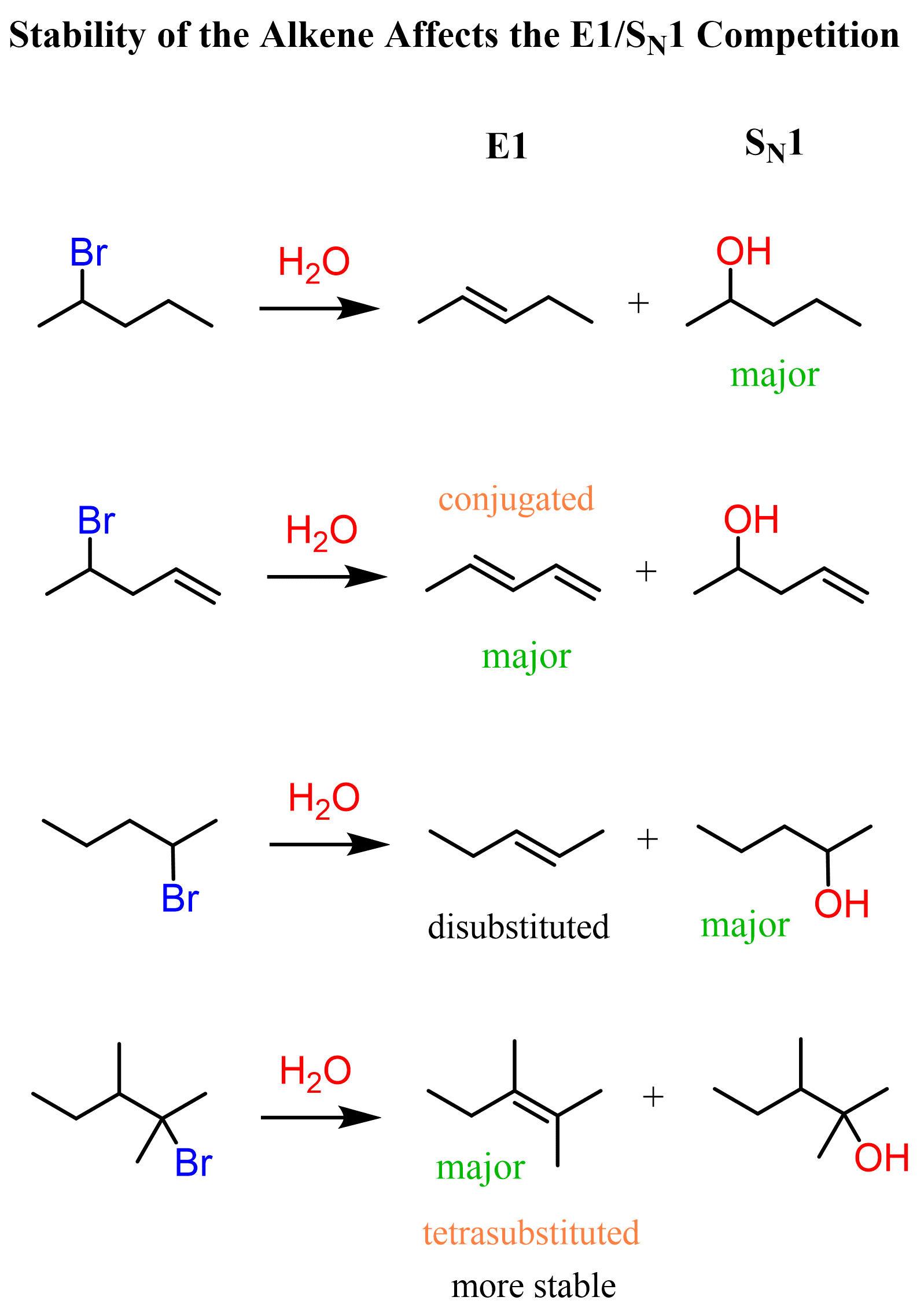
We have just been talking about the SN1 mechanism, so let’s first recall that it is similar to E1 as both proceed via formation of a carbocation intermediate. Therefore, polar protic solvents are favored for E1 reactions, as they stabilize the carbocation intermediate and the leaving group via ion-dipole interactions and hydrogen bonding, just like in SN1.
Remember, very often, a mixture of products is formed, and there is no universal, clear-cut cut between the E1 and SN1 reactions. They only differ in that instead of a nucleophilic attack, the water now acts as a base, removing the β-hydrogen:

The E1 path is most often favored by heating the reaction, so whenever “Heat” is mentioned, that should be a hint for you that your instructor expects E1 to be the major product.
Another factor that favors elimination is the formation of substituted and conjugated alkenes (dienes). Recall that the stability of alkenes increases with the number of alkyl groups on the double bond, and conjugated systems are also associated with additional stabilization.
Check this article for more details and examples on choosing between E1 and SN1 reactions.
The Effect of Solvent in E2 Reactions
E2 is similar to SN2, therefore, using a polar aprotic solvent is preferred because it does not cage the base.
However, the use of polar protic solvents is not as detrimental in suppressing the reactivity of strong bases in E2 reactions as it is for the nucleophilicity in SN2 reactions.
The reason for this is that in SN2, the nucleophile must directly attack the electrophilic carbon, which is often sterically hindered, especially in secondary alkyl halides. If the nucleophile is further solvated (caged) by hydrogen bonding in polar protic solvents, it struggles even more to access that crowded carbon, slowing down or completely preventing the reaction.
In E2, however, the base doesn’t attack a carbon – instead, it abstracts a relatively accessible β-hydrogen, which is less hindered than the electrophilic carbon.

Notice that even if the base is bulky or partially solvated, it can still reach that small hydrogen. That’s why E2 reactions can proceed efficiently even with large, hindered bases or in solvents where SN2 would be suppressed. In short, steric hindrance and solvation affect SN2 more severely because of the nature of the site the nucleophile must attack.
The Effect of Solvent and Ionic Size
We have seen that nucleophilicity is influenced not only by the structure of the nucleophile, but also by the solvent and the size of the ion. In polar protic solvents, nucleophiles are solvated through hydrogen bonding, which reduces their ability to attack electrophilic centers.
For example, fluoride ion, being the smallest and most electronegative, makes strong hydrogen bonds and is tightly caged by the protic solvent molecules.
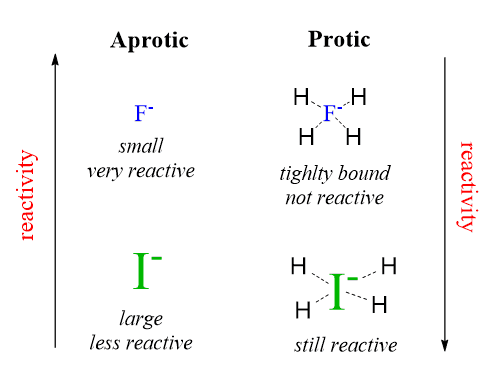
Notice that this effect is more pronounced for smaller anions like F⁻, as they get caged more tightly by protic solvent molecules. In contrast, larger anions such as I⁻ are less solvated and remain more nucleophilic. Therefore:
- In polar protic solvents, nucleophilicity increases down the group: I⁻ > Br⁻ > Cl⁻ > F⁻ (middle column).
- In polar aprotic solvents, solvation is minimal, so basicity and nucleophilicity trends become more aligned – smaller ions are stronger bases and better nucleophiles, so the order is reversed: F⁻ > Cl⁻ > Br⁻ > I⁻

Summary: Solvent Effects in Substitution and Elimination Reactions
Let’s wrap this discussion on polar protic and aprotic solvents by briefly mentioning the key points you need to know:
🔵 Polar aprotic solvents favor SN2 because they do not hydrogen bond with the nucleophile, allowing it to remain reactive.
🔴 Polar protic solvents favor SN1 because they stabilize both the carbocation and the leaving group via hydrogen bonding and ion-dipole interactions.
🟢 Hydrogen bonding suppresses nucleophilicity by caging the nucleophile.
🟢 The caging effect is more detrimental for SN2 than for E2, because SN2 requires a direct attack on a crowded carbon, while E2 only abstracts a β-hydrogen.
🟢 In polar protic solvents, nucleophilicity increases down the group: I⁻ > Br⁻ > Cl⁻ > F⁻
🟢 In polar aprotic solvents, solvation is minimal, so basicity and nucleophilicity trends align and the order is reversed: F⁻ > Cl⁻ > Br⁻ > I⁻
🟢 Both SN1 and E1 mechanisms proceed via a carbocation intermediate and are favored by polar protic solvents.
🟢 SN2 and E2 mechanisms involve concerted steps and are favored by polar aprotic solvents, although solvent effects are less critical in E2.
Choosing Between SN1 and E1 reactions
🟣 Solvolysis reactions involve the solvent acting as both the nucleophile and solvent, common in SN1.
🟣 Heating a reaction tends to favor E1 elimination over SN1 substitution.
🟣 In E1, elimination leads to more substituted and/or conjugated alkenes, which are thermodynamically more stable.