In the previous post, we talked about the orientation effects in EAS when there is more than one substituent on the benzene ring.
In short, to predict the substitution site, you need to determine the stronger activator.
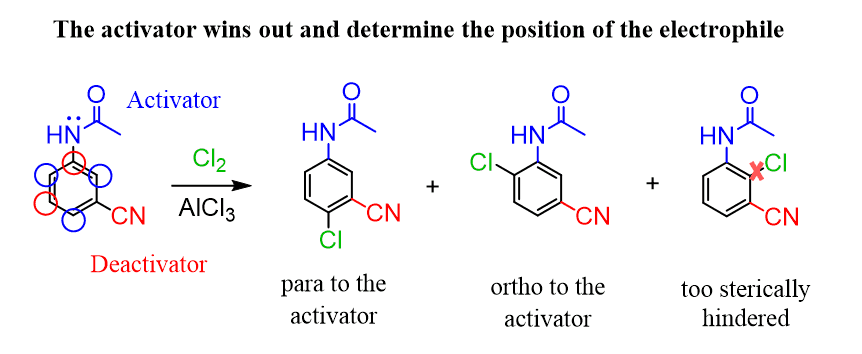
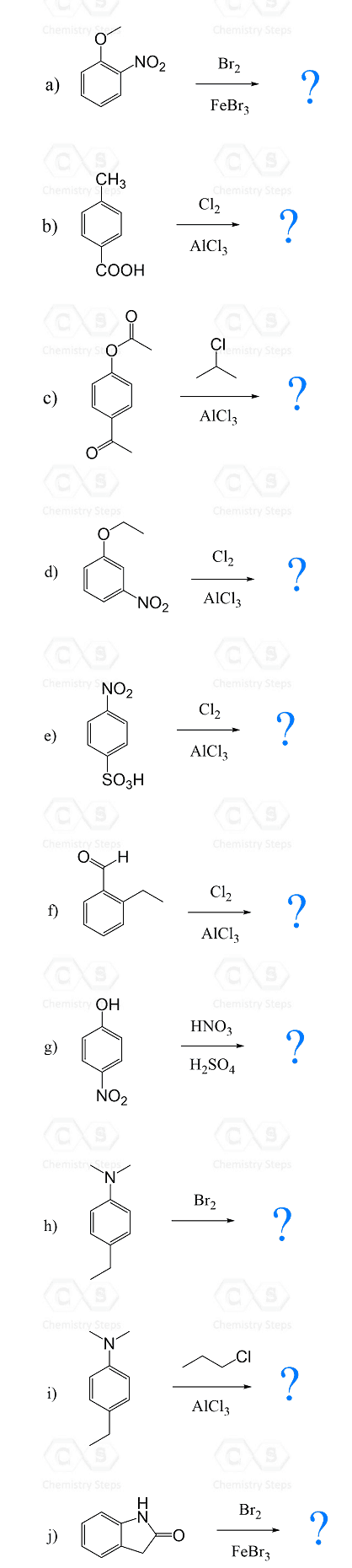
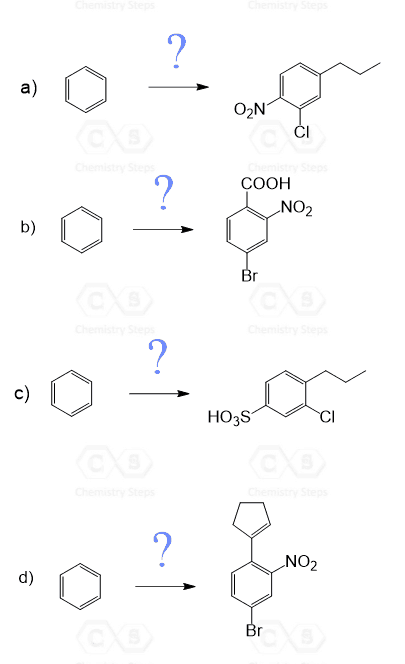
Using this principle, try solving the following practice problems.

In problem 1, part d) I got that one substituent is a strong activator while the other is a strong deactivator, why does the activator take precedence over the deactivator? Wouldn’t placing the Cl on the meta position (relative to the nitrogen group) give less steric hindrance? Any explanation would be appreciated!!
Ok, so the first question can be answered by referring to the ortho/para vs meta directing effects which is the electronic component. Generally, all the activators are ortho/para directors because they enhance the electron density on these positions which makes them more nucleophilic and stabilizes the positive charge in the sigma complex through resonance contribution. Check this post for more details.
For the second question, we need to consider both the electronic and steric effects. The consideration about the steric effect is correct and yes, if the substituent ended up on the meta position relative to the nitro and the methoxy groups, it would be beneficial from the sterics perspective. However, that carbon is barely electron-rich enough to attack the substituent and if it did, the sigma complex would be too unstable since the positive charge of the ring, in that case, cannot be stabilized by the inductive and resonance stabilizing effects of the electron-donating methoxy group.