We have learned the R and S and IUPAC nomenclature rules, and now it’s time to see how these two work together. In other words, how do we name a compound that contains a chiral center?
The idea is simple: we name the compound as we normally do, as if there are no chiral centers, and then, after determining the absolute configurations, we add them with the corresponding locants before the name.
For example, let’s name this alkyl halide following the steps we learned for UPAC nomenclature:
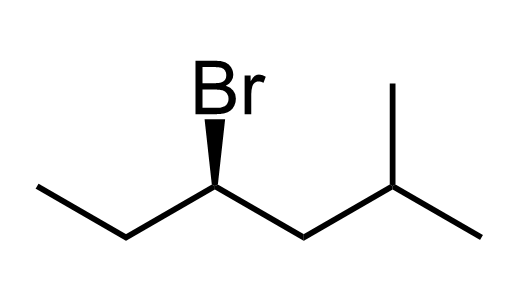
Let’s first determine the parent chain, number the carbons, and assign the R and S configuration.
- Six-carbons is the longest continuous chain; therefore, hexane is the parent chain.
- Next, we number the chain so that substituents get the lowest possible locants: methyl on carbon 2, bromine on carbon 4; therefore, we have 4-bromo-2-methylhexane. Notice that numbering from the other end would give 3-bromo-5-methyl, which has higher locants, thus not correct according to the IUPAC rules.
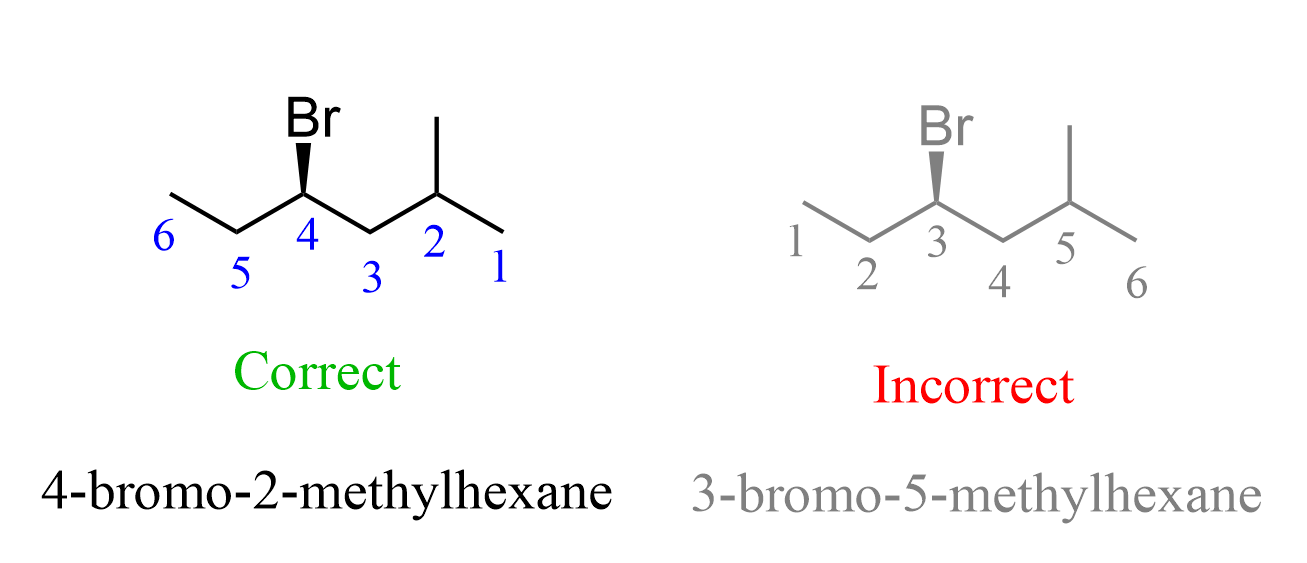
3) Determine the chiral center – carbon 4 is attached to four different groups: Br, H, ethyl, and the remaining chain, and it is the only chiral center in the molecule.
4) Assign priorities according to Cahn–Ingold–Prelog rules:
We first look at the atoms directly attached to the chiral center. Br is priority one; however, there are CH₂ groups on both sides of the carbon, so to determine priority 2, we move to the next set of atoms.
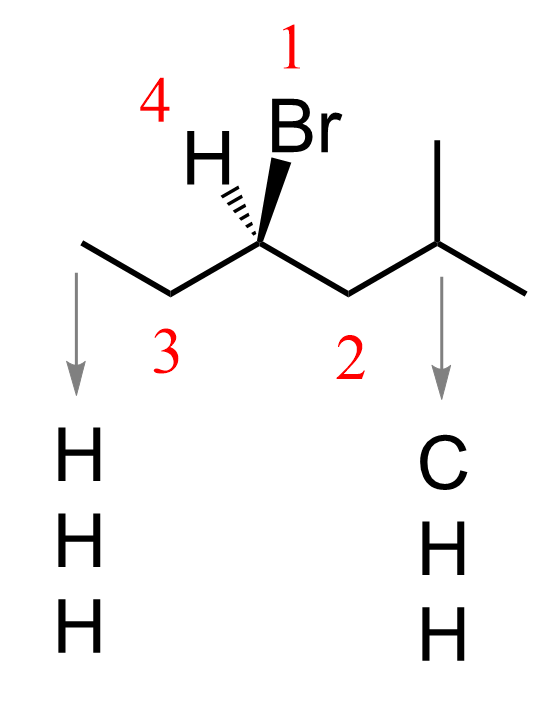
The next carbon on the left (CH₃) is connected to three hydrogens, whereas the one on the right is connected to a carbon and two hydrogens; therefore, the CH₂ on the right gets priority 2. The lowest priority, hydrogen, is pointing away from us, and the arrow goes clockwise; therefore, the absolute configuration is R.
We add it with the corresponding locant in parentheses before the rest; therefore, the name is (R)-4-bromo-2-methylhexane.
Although not often, sometimes your professor may show an achiral carbon with a wedge or dash bond to make it a little trickier.
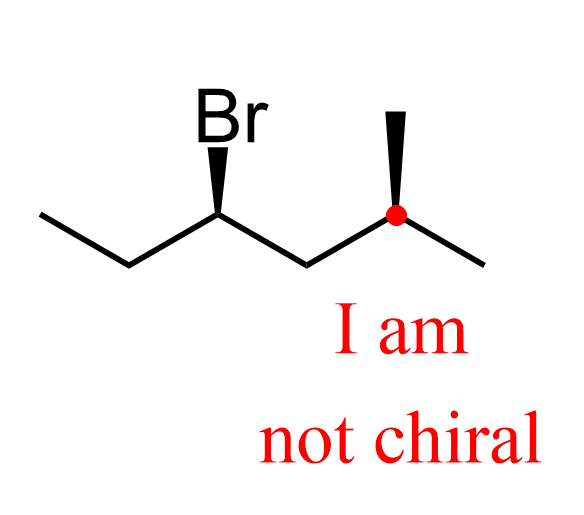
Do not try to determine the absolute configuration of carbon 2 if the methyl is shown with a wedge or dash – it’s not chiral.
Naming a Molecule with Two Chiral Centers
Now let’s do another example, this time, a cyclic structure with two chiral centers:
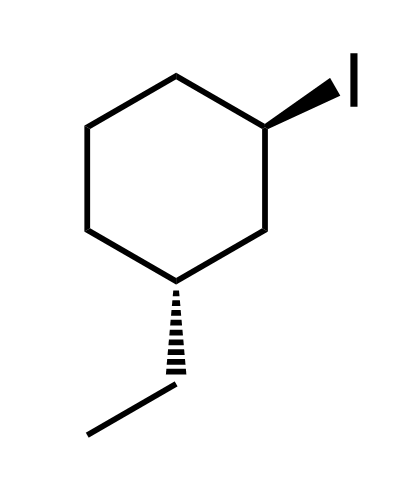
- The parent structure is cyclohexane.
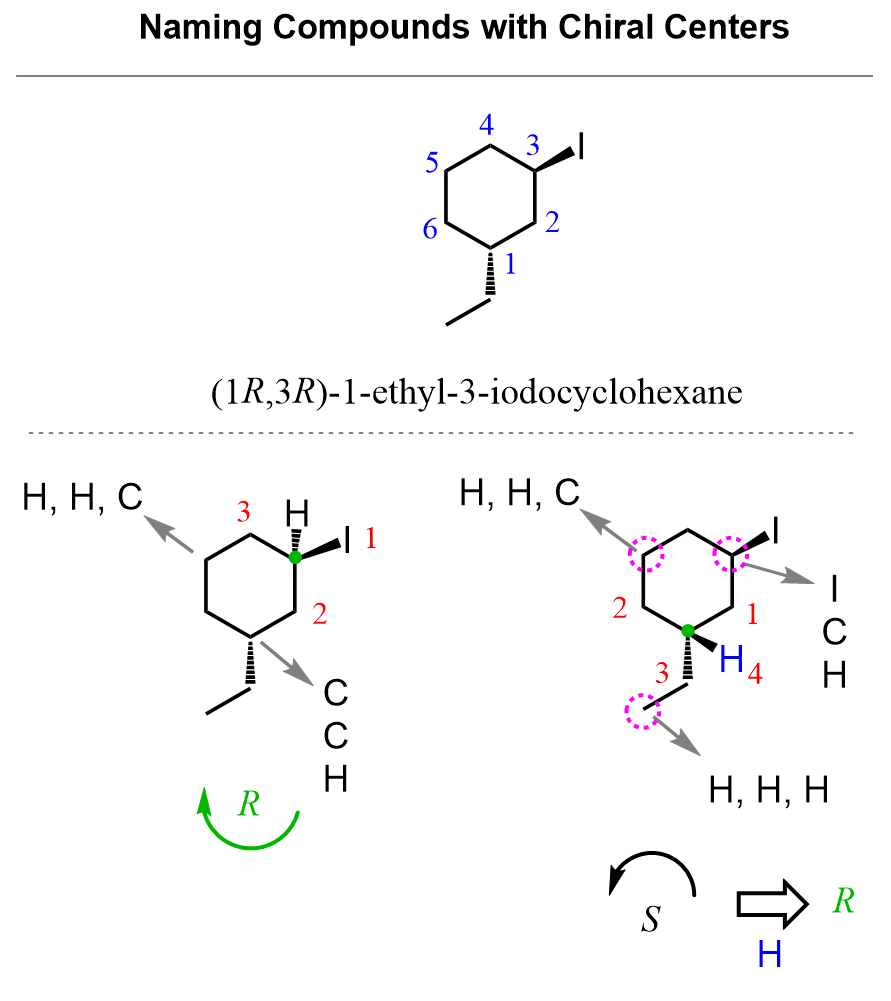
- Substituents are on carbons 1 and 3: an ethyl and an iodo group, so we have 1-ethyl-3-iodocyclohexane.
Remember that halogens have no functional group priority, so do not automatically start numbering at the carbon connected to the halogen. In this case, for giving the lowest possible locants, it does not matter where we start numbering – it will be 1,3 either way. However, because of alphabetical order, “ethyl” comes before “iodo,” so numbering starts at the ethyl.
- Determine absolute configurations for both chiral centers – both are R.
On both chiral centers, there are CH₂ groups, which means we continue to the next atoms to find the tie break. The first one is easier because we have an iodine, and it clearly gets priority 1. For the second chiral center, we are comparing the atoms connected to the three CH₂ groups (circled in purple) that are directly connected to the chiral center (first layer). So, we skip them and compare the carbon atoms connected to them.
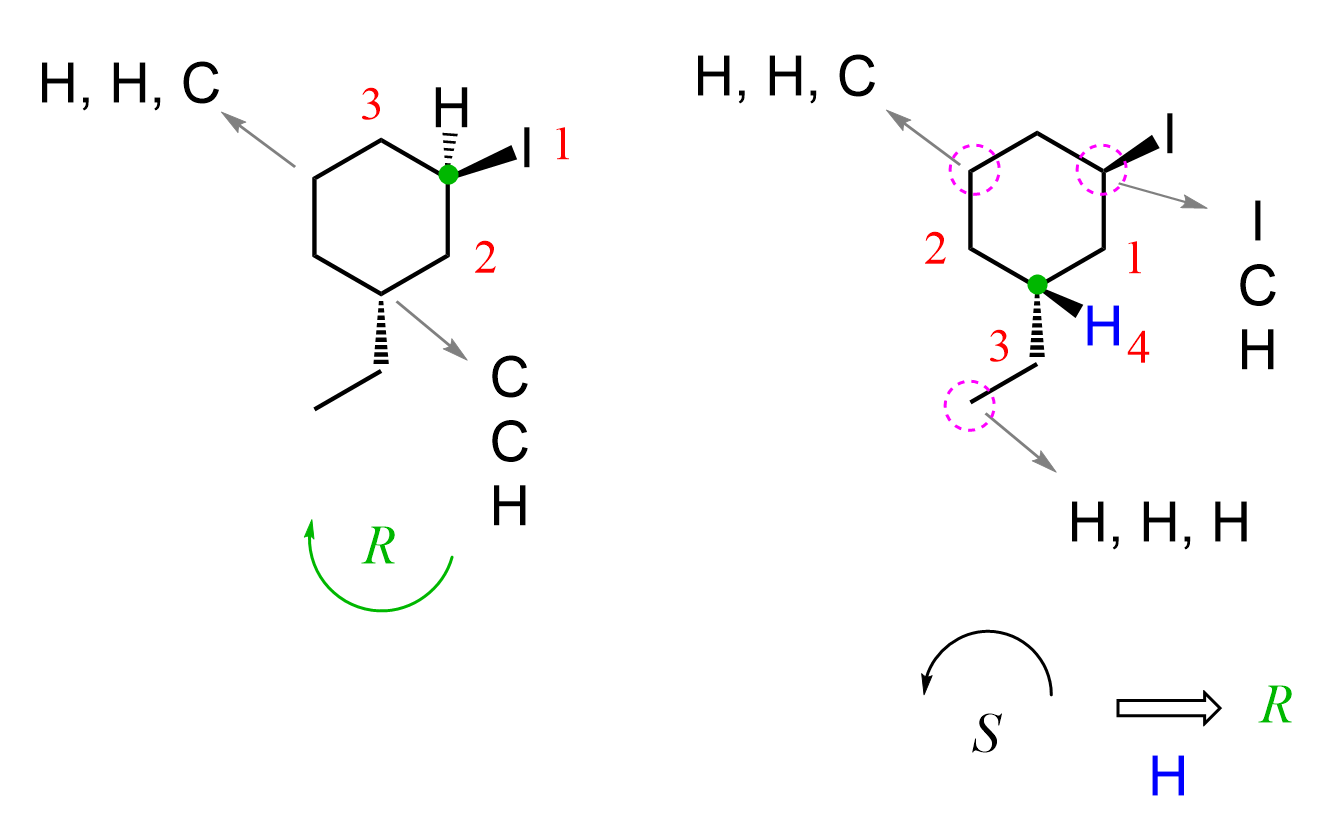
The one on the right side gets priority 1 because of the iodine, and the one on the left side is priority two, as it is a CH2 group competing with a CH3 on the bottom. The arrow goes counterclockwise, which indicates S; however, because the hydrogen is pointing towards us, we switch it to R. The final name is (1R,3R)-1-ethyl-3-iodocyclohexane.
E and Z Notation in Nomenclature
Let’s also consider a molecule which, in addition to chiral centers, has a double bond:
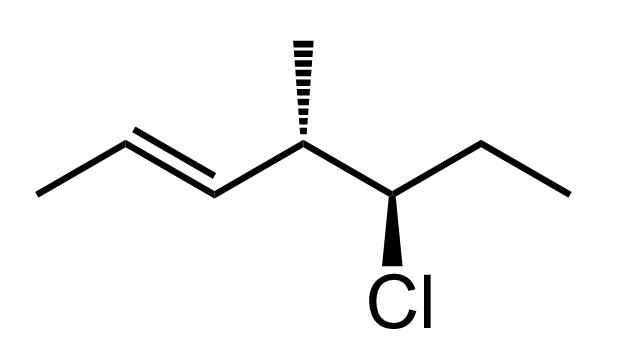
The parent chain of this molecule is a seven-carbon chain, so the base name will be heptane. When numbering the chain, we always start from the end closest to the double bond so that the double bond gets the lowest possible locant.
So far, we have 5-chloro-4-methylhept-2-ene:
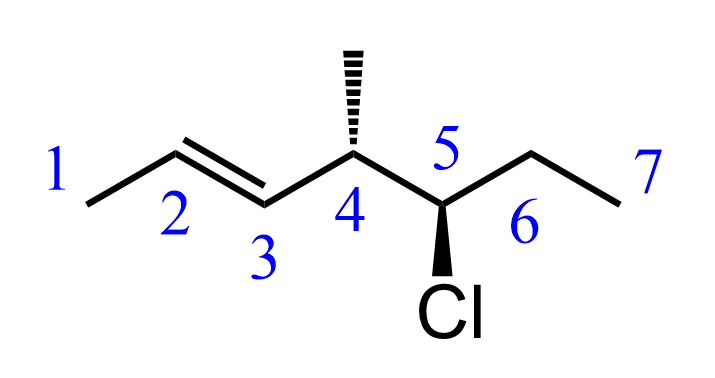
Next, we determine the configuration of the double bond.
Remember that double bonds are stereoisomeric if none of the carbons is connected to two identical groups. Both carbons of the double bond in this molecule are connected to hydrogen and carbon atoms, so we need to determine the E/Z configuration and add it to the name of the molecule.
A quick reminder on determining E and Z configurations.
- The configuration of the double bond is E when the highest-priority groups on each carbon are on opposite sides.
- It is Z when the highest-priority groups on each carbon are on the same side.

In this molecule, the hydrogens on both carbons are pointing in opposite directions; therefore, the configuration is E. We can also say that the higher priority groups – the CH3 and the carbon chain are on the opposite sides of the double which indicates an E configuration.
Let’s also determine the absolute configurations, starting with carbon 4, and combine everything to get the full name of the molecule.
The first priority is the carbon connected to chlorine, followed by the double-bonded carbon, and the methyl group is priority 3. The arrow goes clockwise; however, because of the wedge hydrogen, we switch it to S.
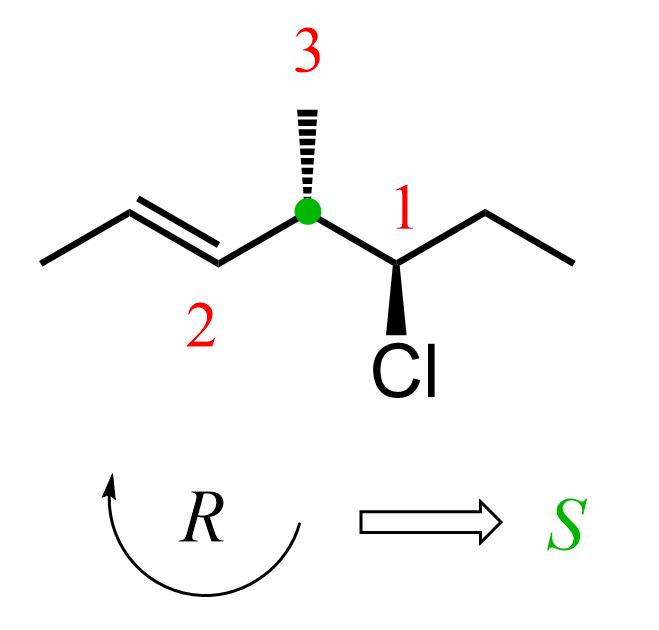
On carbon 5, chlorine is the first priority, followed by the carbon on the left since it is connected to two carbon atoms, and the ethyl group is priority 3. The arrow goes clockwise; therefore, the absolute configuration is R.
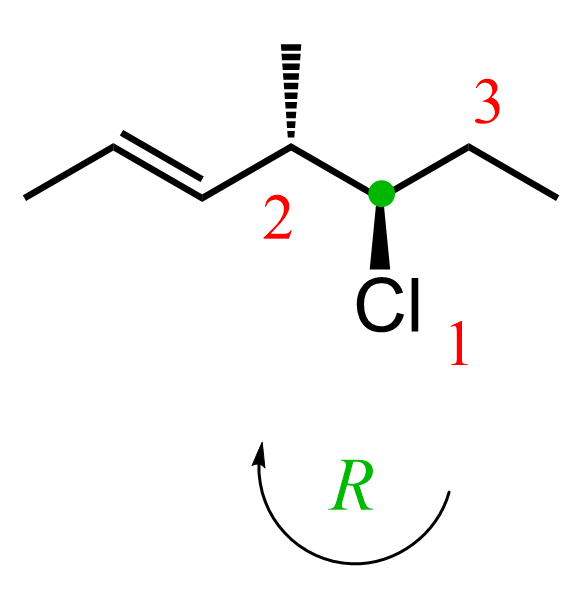
Just like R and S, E and Z are added to the name in parentheses with the corresponding locants; therefore, the name is (4S,5R,E)-5-chloro-4-methylhept-2-ene.
Summary: Naming Chiral Compounds
When naming compounds with chiral or stereogenic centers, the key idea is simple — start by naming the molecule as if it had no chiral centers or double-bond stereochemistry. Follow all the regular IUPAC rules for identifying the parent chain, numbering, and naming substituents. Once the base name is complete, determine the absolute configurations (R/S) for each chiral center and E/Z configurations for any double bonds.
After assigning them, write the configurations in parentheses, with the corresponding locants, before the main name of the compound.
For example:
- A single chiral center – (R)-4-bromo-2-methylhexane
- Two chiral centers – (1R,3R)-1-ethyl-3-iodocyclohexane
- Both chiral centers and a double bond – (4S,5R,E)-5-chloro-4-methylhept-2-ene
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- IUPAC Nomenclature Summary Quiz
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- Cis and Trans Isomers
- E and Z Alkene Configuration with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
- Stereochemistry Practice Problems
- Stereochemistry Practice Quiz
- Nomenclature of Alkyl Halides
- Naming Alkenes by IUPAC Nomenclature Rules
