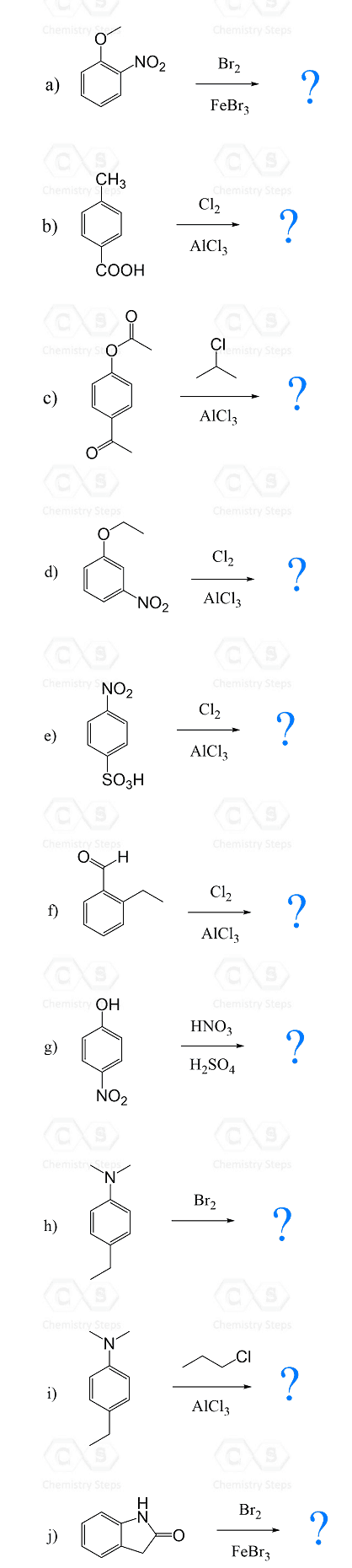
In the previous post, we saw that a benzene ring with an activator undergoes electrophilic aromatic substitution at the ortho and para positions, while deactivated aromatic rings react at the meta position:

Here is an interesting question: Where does the substitution occur if there are two groups on the benzene ring (disubstituted benzene)?
There are two main possibilities. The first one is when both groups are directing the electrophile to the same position. This is straightforward since there is no conflict:
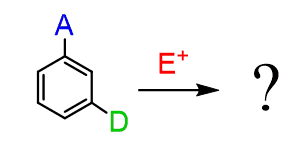
The second would be a reaction of a ring with where the two groups orient the electrophile to different positions:
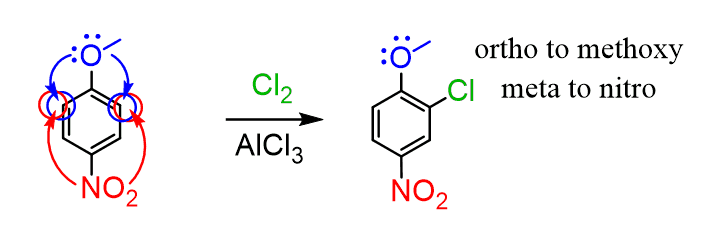
In this case, the stronger activator determines the position of the electrophilic substitution:
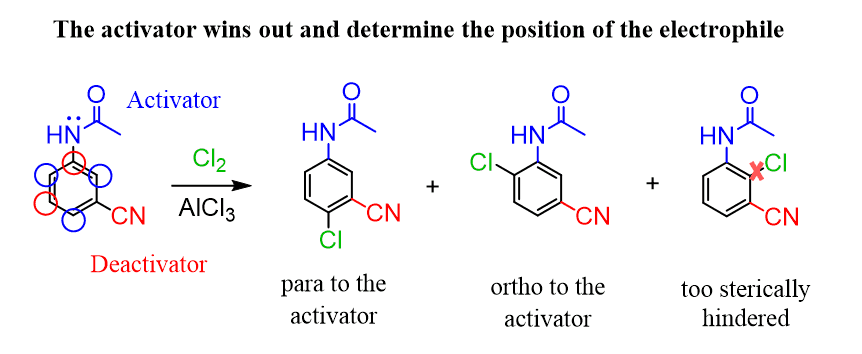
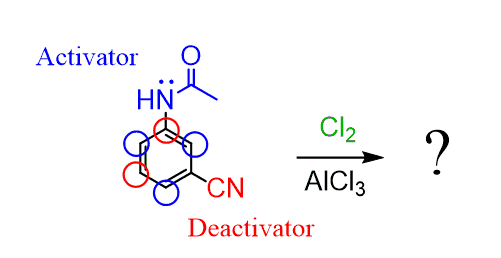
The nitrogen is an activator while the cyano group is a strong deactivator. Therefore, the electrophile is directed by the acylated amino group to its ortho and para positions.
Keep in mind also the steric considerations. For example, substitution does not occur between two meta substituents and in general, the electrophile goes to the least hindered position.
Even a weak activator “wins out” the battle with a moderate or strong deactivator and if there are two activators then the stronger activator defines the Regiochemistry of the substitution reaction:

Another possibility is the combination of two deactivators. The first thing here is to remember that not all the electrophilic substitution reactions occur on deactivated benzene rings. For example, Friedel-Crafts reactions do not work with deactivated aromatic rings:
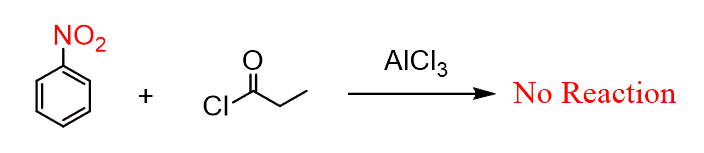
However, if the reaction does occur, then there is not regioselectivity and a mixture of products is obtained.
In short, any group standing higher in this table determine the position of the electrophilic aromatic substitution of a disubstituted benzene ring: