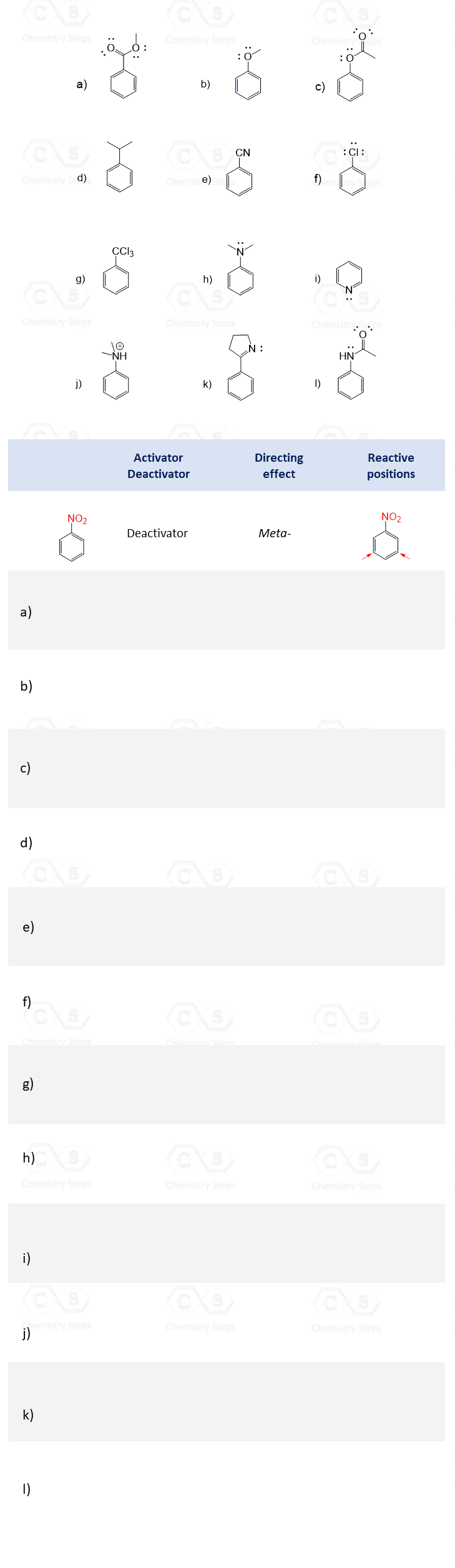
We have seen earlier that in electrophilic aromatic substitutions, it is the benzene ring that provides the electrons to react with electron-deficient species – electrophiles.
Therefore, the more electron density we supply to benzene, the more reactive it becomes in EAS reactions.
The groups that provide electron density to benzene are classified as activating groups, and those that withdraw electron density are consequently known as deactivating groups or deactivators.
So, what types of electron-donating groups do we know?
Let’s recall the stability of carbocations, which increases with the number of alkyl groups connected to the positively charged carbon atom.

Therefore, alkyl groups are electron donors. To be more specific, they are electron donors via the inductive effect. As a reminder, the inductive effect is the electron-donating or -withdrawing effect transmitted through sigma bonds due to differences in electronegativity of atoms or groups, which influences the electron density on adjacent atoms.
We are going to talk about inductive and resonance effects a lot today, so let’s put this list here as a reference point:
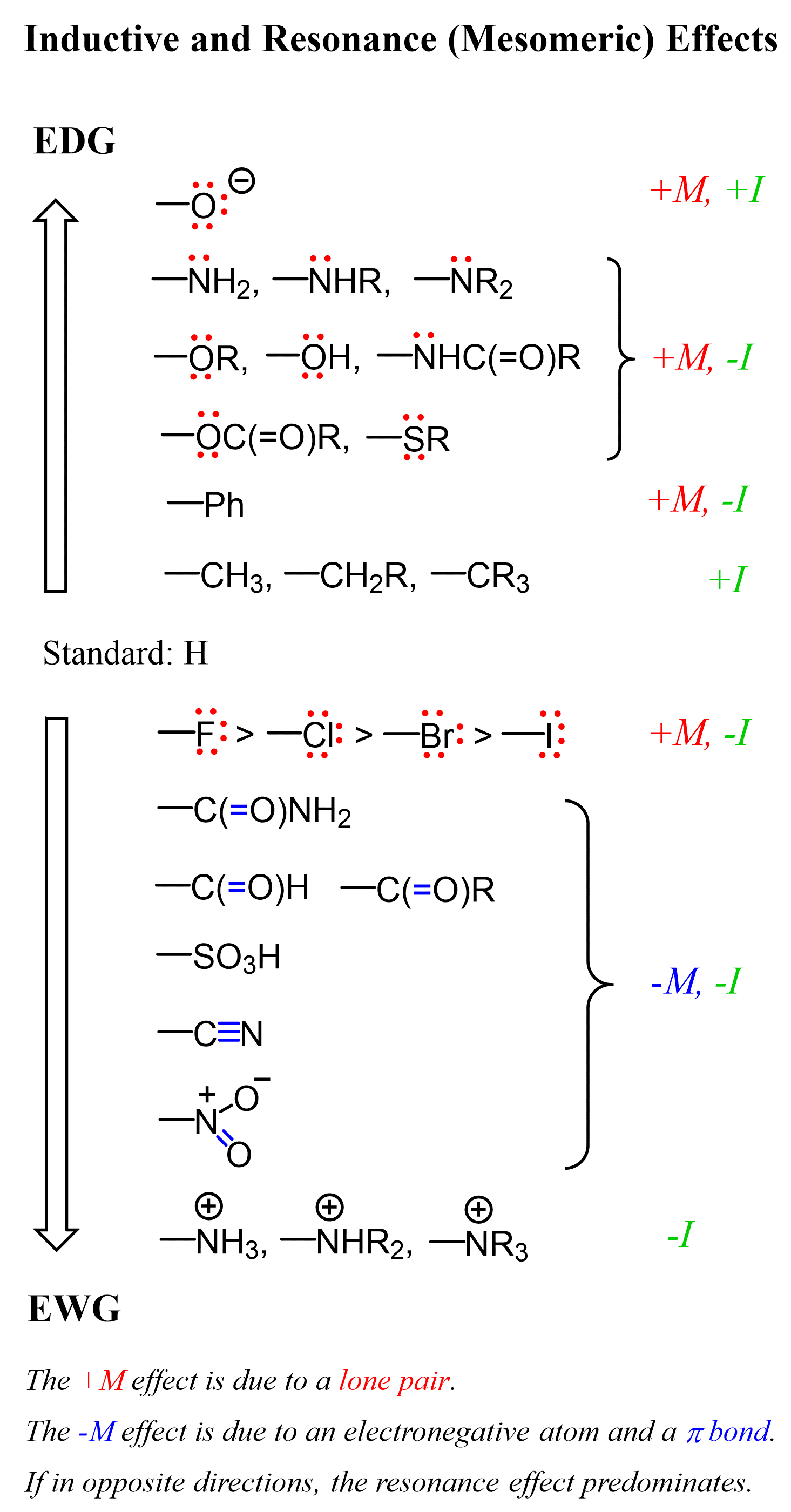
Activation via Inductive Effect
Keeping in mind that alkyl halides are electron donors, what do you think – would benzene or toluene react faster in electrophilic aromatic substitution reactions?
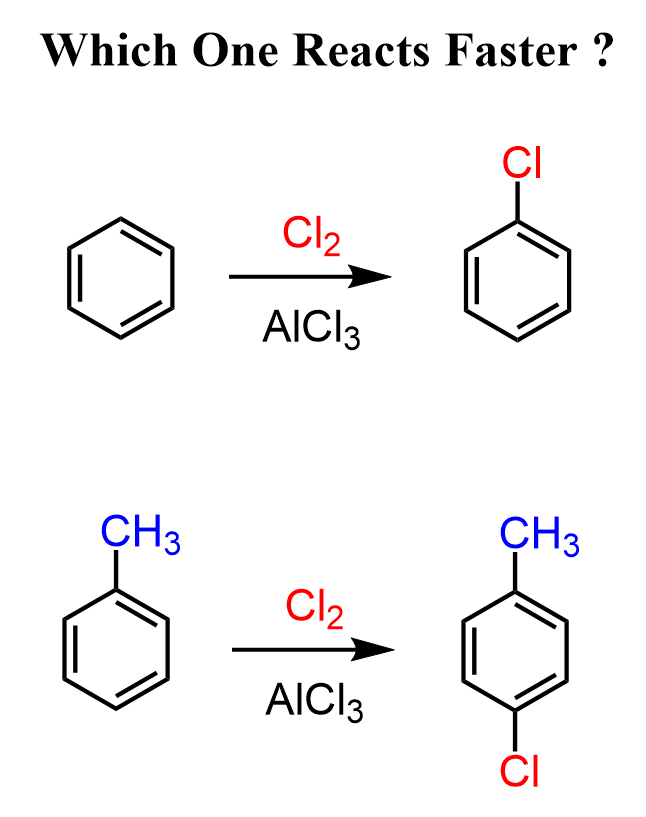
Hopefully, your answer is toluene because, once again, alkyl groups are electron donors and they make the aromatic ring more electron-rich, thus more reactive in EAS reactions.
We can confirm this by experimental data of the chlorination of benzene and toluene under identical conditions. The chlorination of toluene proceeds about 30 times faster than benzene.
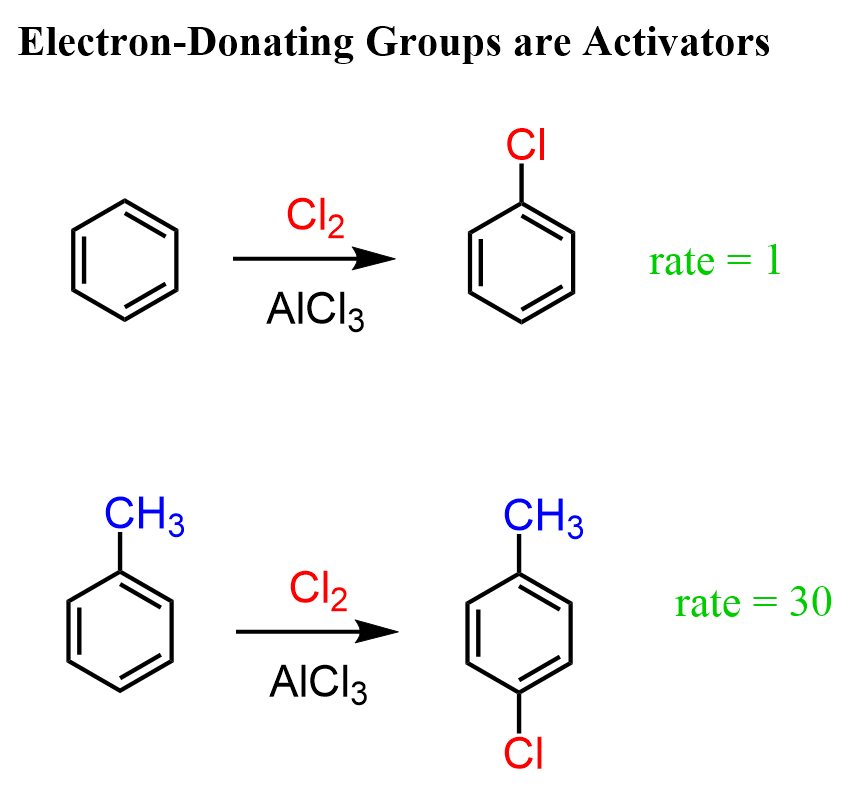
This significant rate increase clearly shows the activating effect of the methyl group on the aromatic ring during electrophilic aromatic substitution.
Resonance-Activating Groups in EAS
Let’s now see how resonance-donating groups affect the reactivity in EAS reactions. A quick reminder: resonance donation occurs when a substituent atom or group donates electron density to the aromatic ring through the overlap of its lone pair electrons with the π system of the ring. This electron donation makes the ring more electron-rich, thus more reactive toward electrophiles.
As we compared the reactivity of benzene with toluene earlier, let’s now compare benzene and anisole (methoxybenzene). Anisole differs from toluene by the presence of an oxygen atom linking the methyl group to the aromatic ring, and this oxygen makes a big difference. Experimental data show that the rate of chlorination of anisole is approximately 10⁴ times faster than that of benzene.
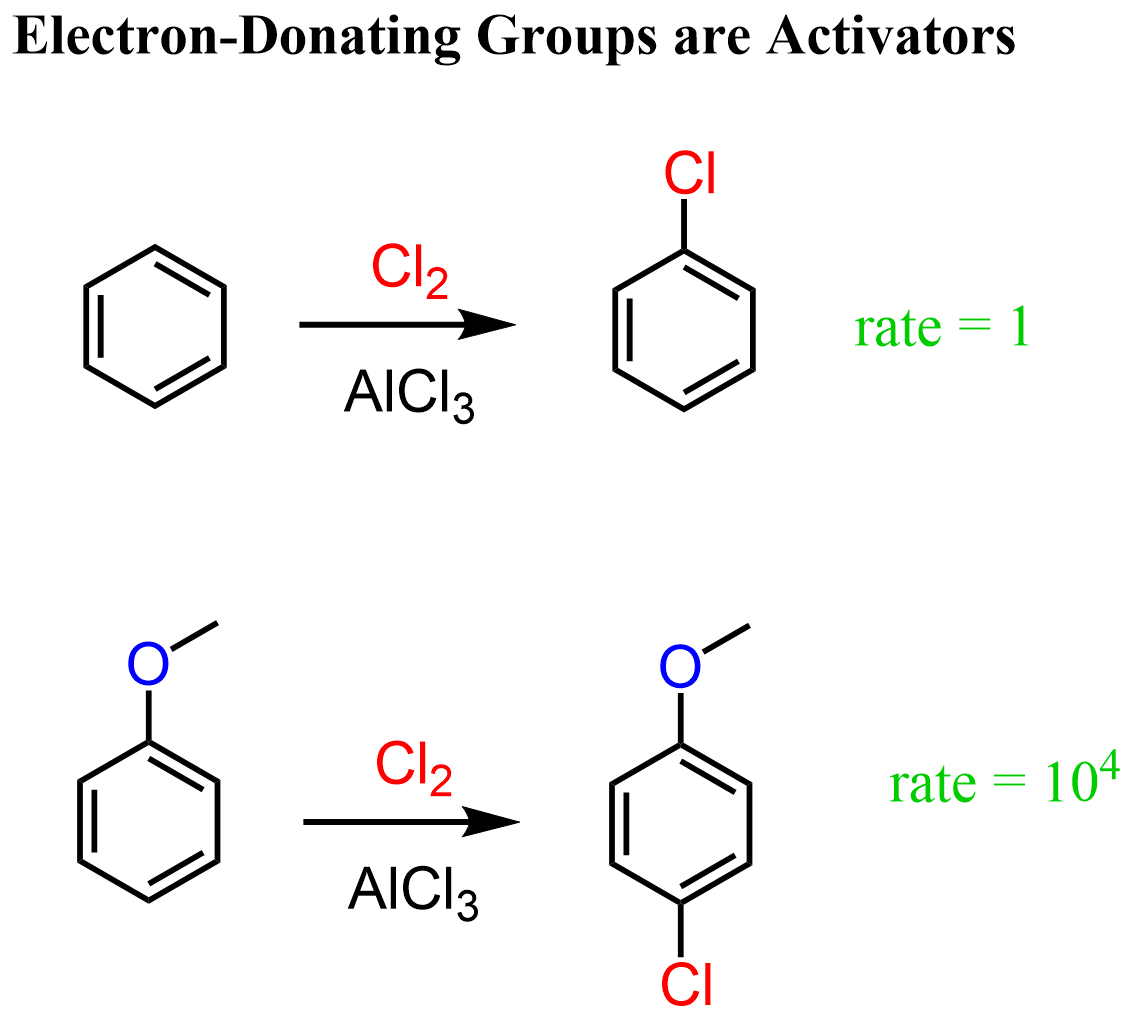
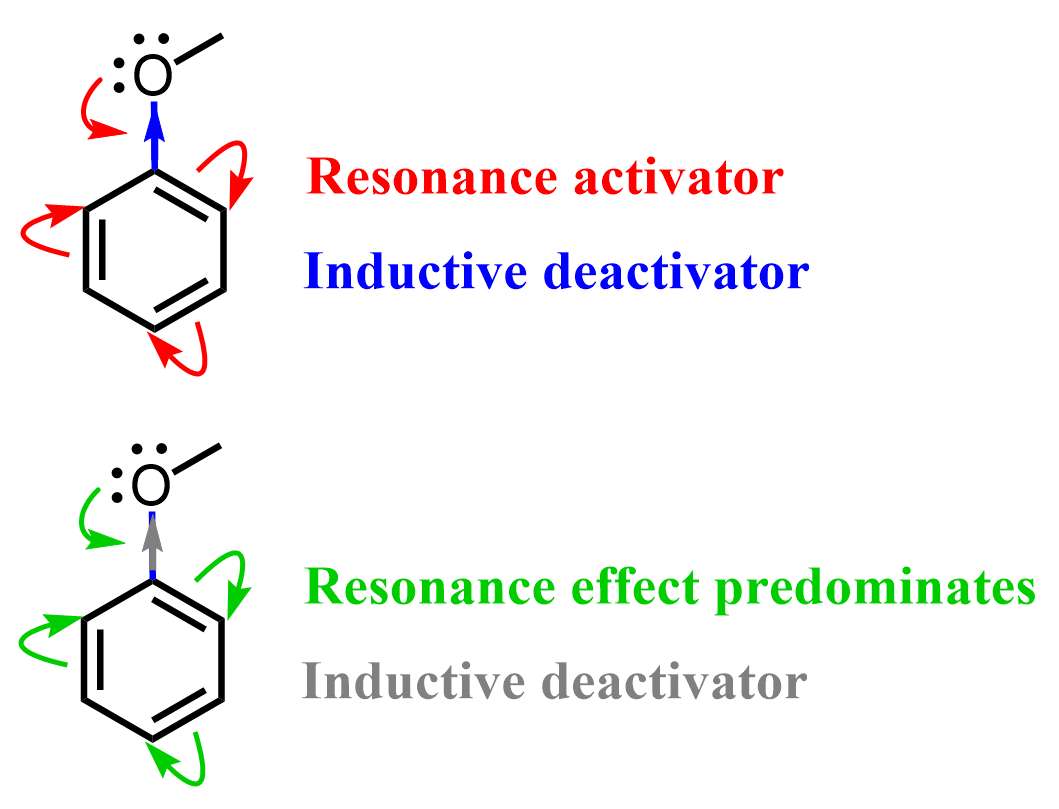
This may seem counterintuitive for a moment because oxygen is more electronegative than carbon, and you may argue that it should withdraw electron density from the ring, making it less reactive. While there is nothing wrong with that argument, you need to also remember that oxygen has lone pairs that are delocalized over the aromatic ring. This is the resonance-donating effect, and it is more predominant than the inductive effect, resulting in an overall increase in electron density on the ring and greatly enhancing its reactivity in EAS reactions.
Resonance Activators and Lewis Acid Catalysts
The effect of resonance-activation is perhaps best seen in the halogenation of phenol and aniline, which undergo halogenation rapidly without the need for Lewis acid catalysts such as AlCl3 or FeBr3:

Nitrogen is closer in size to carbon, allowing better orbital overlap and thus a stronger resonance-donating effect. Because of this strong resonance donation, phenol and especially aniline are extremely reactive compared to many other benzene derivatives.
This delocalization of the lone pair electrons into the aromatic ring stabilizes the intermediate arenium ion formed during electrophilic substitution, significantly increasing the ring’s reactivity toward electrophiles. Their high reactivity usually leads to full halogenation, where multiple halogen atoms substitute onto the ring under mild conditions.
Activating Groups are Ortho/Para Directors
Notice that in all the reactions above, when an activating group was present on the ring, the EAS occurred at the ortho and para positions. So, the question is: why do all activators direct the electrophile to the ortho and para positions?
To understand this, we need to draw the mechanisms of these electrophilic aromatic substitutions. Let’s see how that happens using the example of aniline bromination. The first and last rows show the bromination at the ortho and para positions, while the middle row shows the reaction at the meta position.
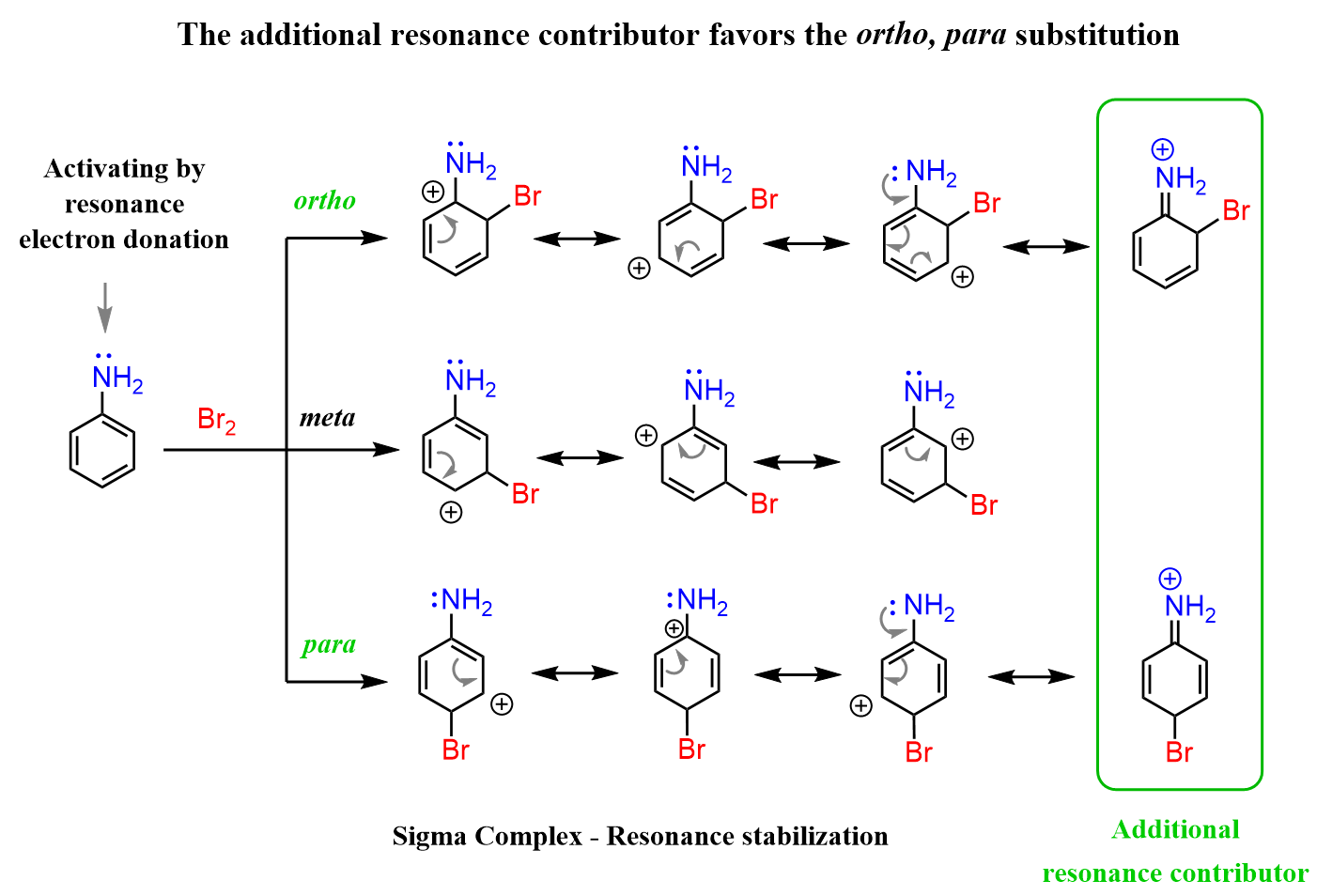
What makes the ortho/para bromination more favorable is the extra resonance stabilization that involves the lone pairs of the nitrogen. This is not possible when the electrophile reacts at the meta position, as the positive charge never ends up next to the nitrogen.
The ortho–para preference is the case in any electrophilic aromatic substitution of resonance-activated benzene derivatives.
A similar pattern is observed when an activator with an inductive effect is present. The only difference is that the positive charge next to the activator is now stabilized via an inductive effect.

This stabilization is not as significant compared to that provided by resonance activators because the inductive donation does not satisfy the octet of the positively charged carbon.
Therefore, the resonance donation is generally stronger and cancels out the reverse inductive effect. Once again, don’t forget that oxygen and nitrogen are more electronegative than carbon, and they deactivate the ring by an inductive effect, which is overwhelmed (canceled) by the stronger resonance-donating effect.
Deactivating Groups
Let’s also talk about deactivating groups in electrophilic aromatic substitutions. These are the groups that slow down the rates of EAS reactions. Based on what we learned about the activating groups, deactivators reduce the electron density of the benzene ring, thus making it less reactive.
What types of electron-withdrawing groups can you think of?
We are looking for groups where the atom connecting to the aromatic ring is electron-deficient. This can be achieved by both inductive and resonance effects. We have seen that heteroatoms such as nitrogen and oxygen are activators, so the next thing that comes to mind is having a carbon with an electron-withdrawing group.
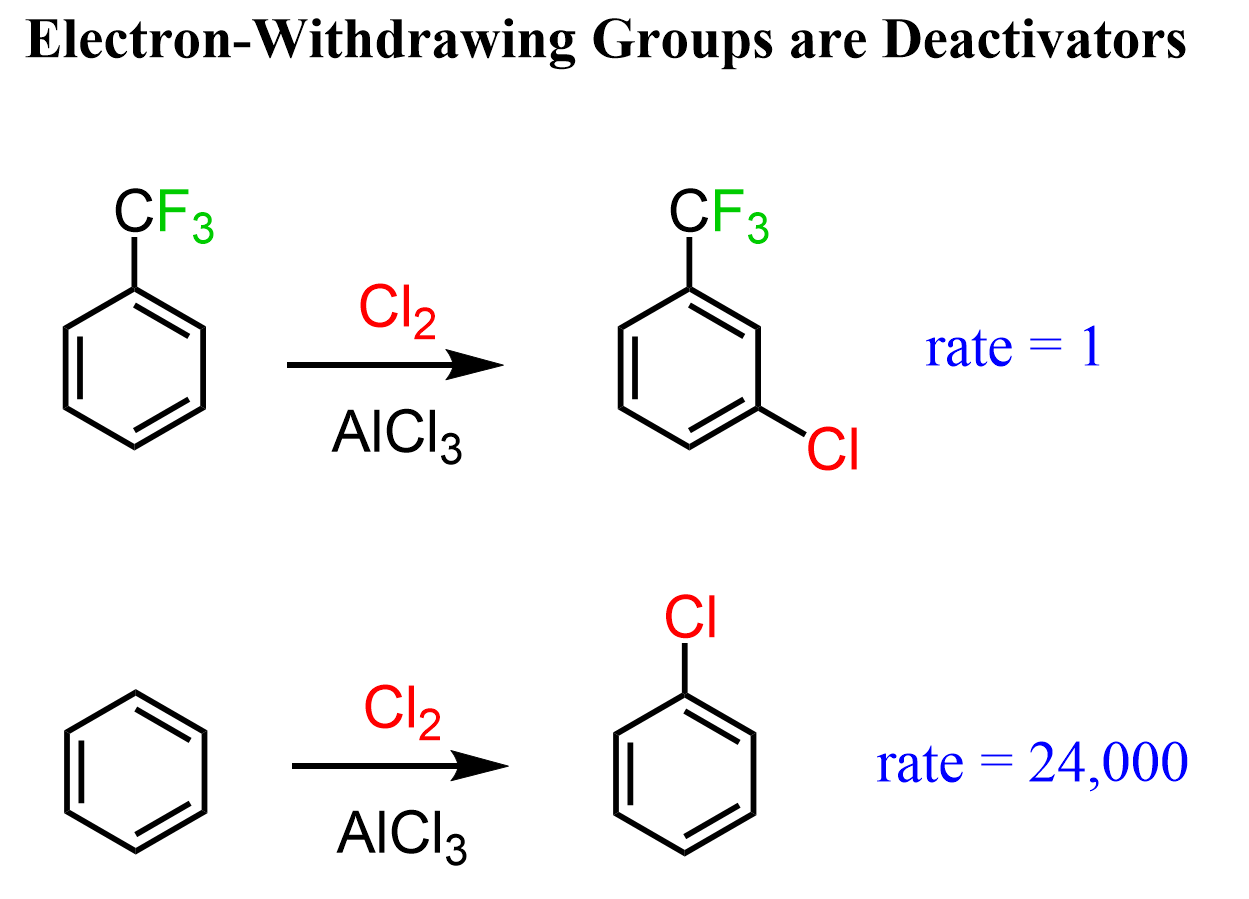
For example, if we replace the three hydrogen atoms of toluene with fluorines, that central carbon becomes very electron-deficient because of the greater electronegativity of fluorine atoms.
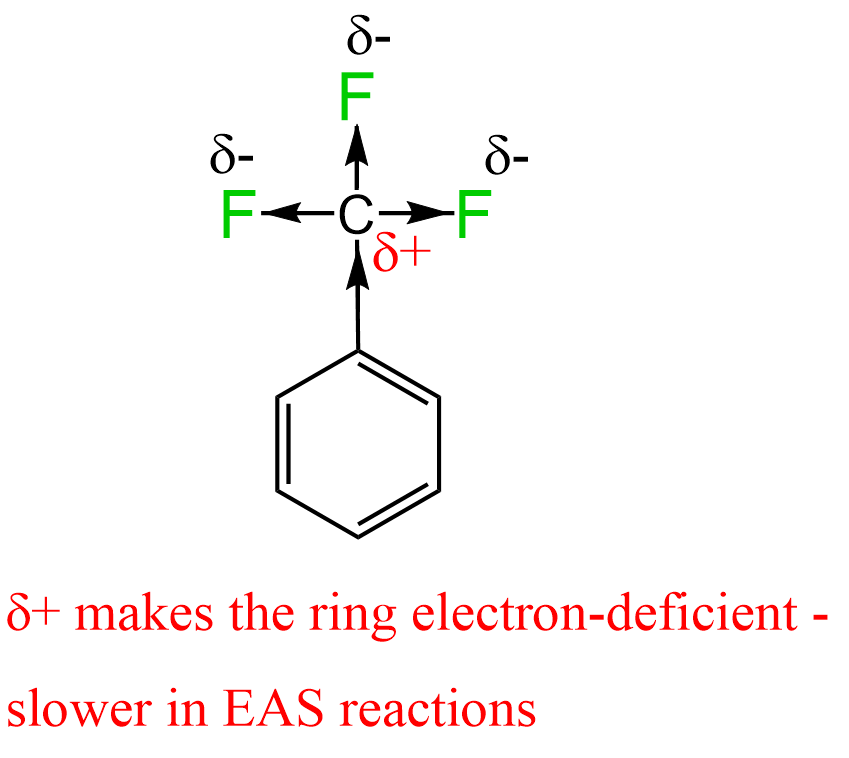
Now, if this group is attached to the benzene ring, it will withdraw significant electron density from the ring, making it less reactive in EAS reactions. As expected, the chlorination of benzene is about 24,000 times faster than that of trifluoromethylbenzene.
Other common deactivators are nitro and carbonyl groups, which slow down the rate of EAS reactions by making it less electron-rich.
At this point, it might be a good idea to put together a list of the most common activating and deactivating groups in EAS reactions:

Deactivating Groups are Meta Directors
Deactivating groups are meta directors, and we can see why by looking at the mechanism of nitration of benzene. For the reaction to occur, the benzene ring attacks the electrophile, forming a carbocation intermediate. When a deactivating group is present, in the case of ortho and para substitution, one of the resonance forms places the positive charge directly next to the electron-withdrawing group.

This is highly destabilizing because the group pulls electron density away from the carbocation center, making the intermediate much less stable and the reaction slower. In the meta position, however, none of the resonance structures puts the positive charge on the carbon directly adjacent to the deactivating group, so this destabilization is avoided. As a result, meta-substitution is favored.
What if an Activating and Deactivating Groups Are Present on the Ring?
If both an activating and a deactivating group are present on the aromatic ring, the position of electrophilic substitution is dictated by the activator, since it donates electron density and stabilizes the carbocation more effectively than the deactivator withdraws it. For example, in the chlorination of a benzene ring bearing an acylated amino group (activator) and a nitro group (deactivator), the major product results from substitution ortho or para to the activator.
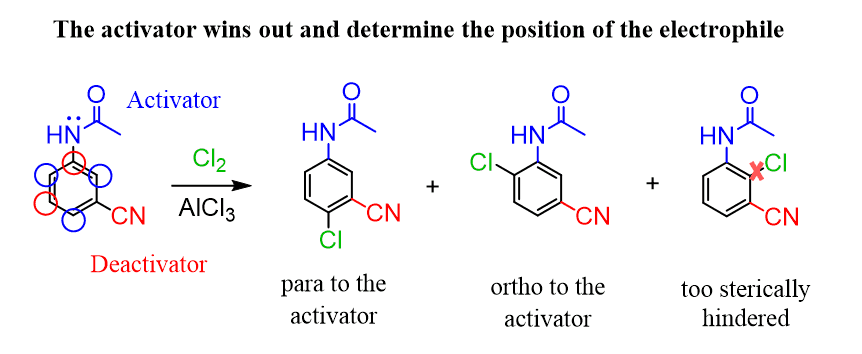
Notice that chlorination ortho to the carbon between the two groups is negligible due to steric hindrance.
Check out this article for more details and examples of EAS on disubstituted benzene derivatives.