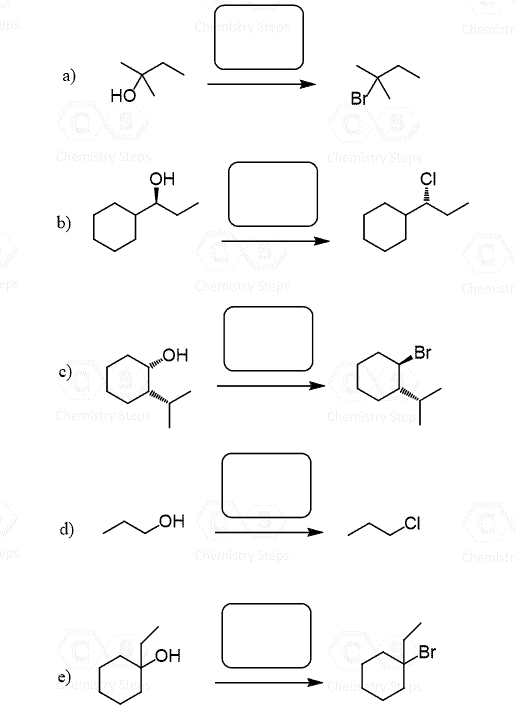
Alcohols can serve both as a nucleophile and an electrophile (substrate) in SN1 and SN2 reactions. However, by themselves, alcohols are not great for any of these reactions because 1) they are weak nucleophiles and 2) the OH is not a leaving group.
There are different ways of making them work, so let’s first put a general scheme for those strategies:
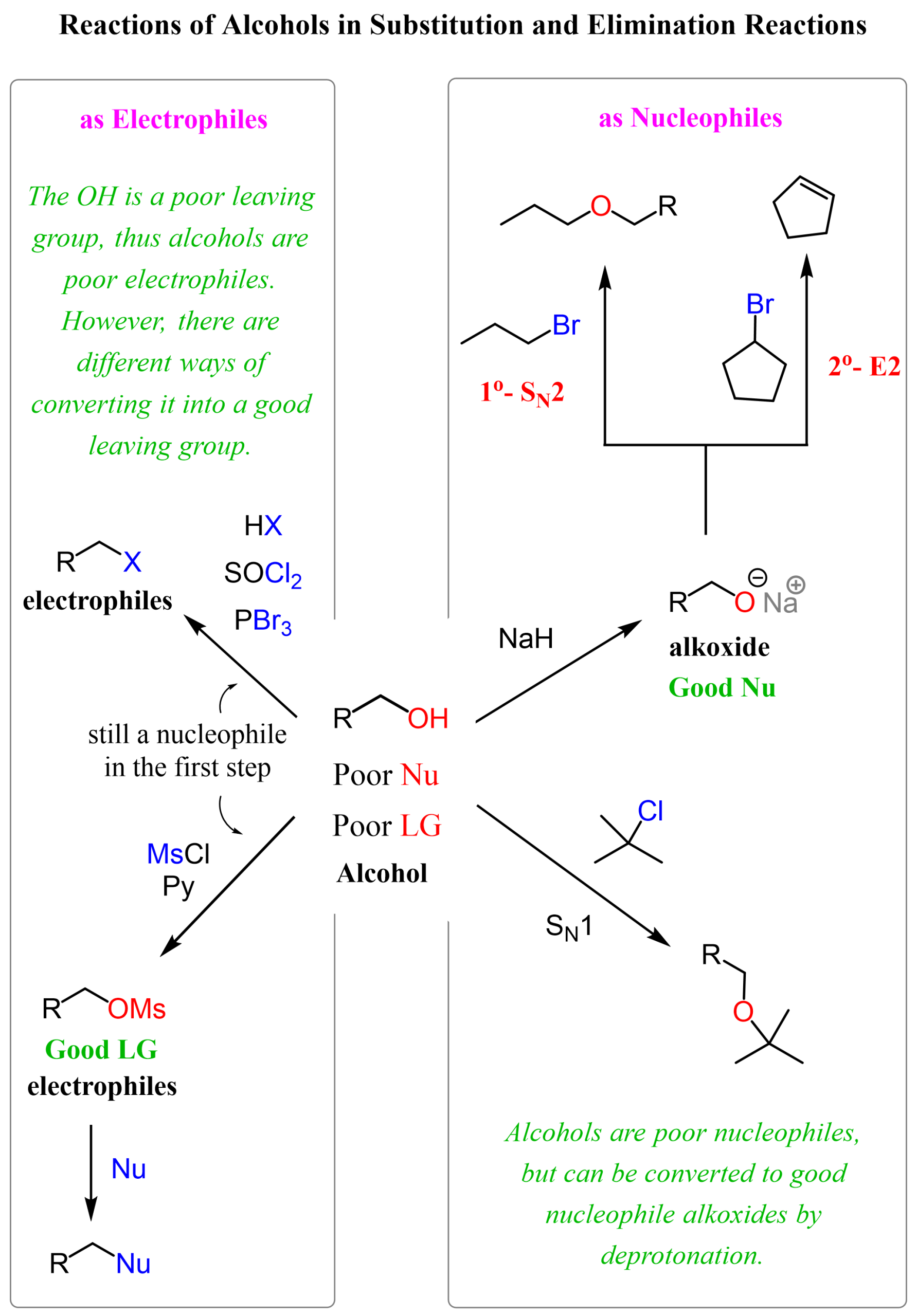
On the right side, we have example reactions where the alcohol reacts as a nucleophile. As mentioned, they are weak nucleophiles, and by themselves, can only participate in SN1 and E1 reactions. So, for making alcohols good nucleophiles, we need to first deprotonate them into the corresponding alkoxides.
To use the alcohol as a substrate (like an alkyl halide), we either protonate the OH group by using HCl, HBr, or HI, and this reaction converts the alcohol into an alkyl halide. To have a larger pool of nucleophiles reacting with alcohols, we convert the OH group into a mesylate, tosylate, or a halogen to act as a good leaving group.
Let’s first discuss the reactions of alcohols as a nucleophile in SN1 and SN2 reactions.
Alcohols as Nucleophiles in SN1 and SN2 Reactions
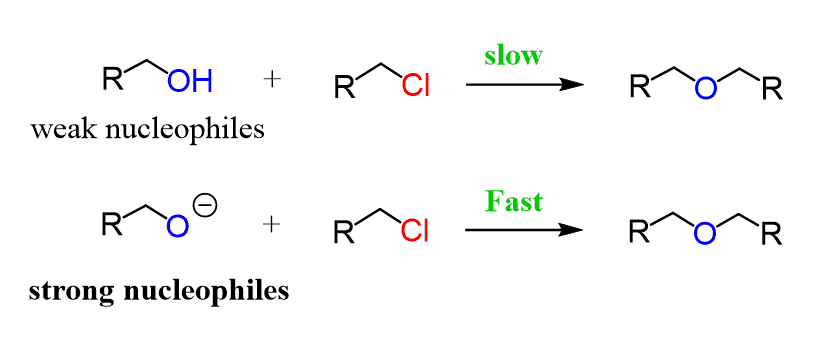
Alcohols together with water are the most common weak nucleophiles; therefore, they only participate in SN1 and E1 reactions. Remember, these are unimolecular reactions that proceed via formation of a carbocation intermediate; therefore, we can only use this strategy for tertiary and secondary alkyl halides.
We have only one example of a primary alkyl halide reacting with an alcohol, so if you wonder why primary alkyl halides are not suitable for SN1, remember that primary carbocations are very unstable; therefore, we can’t use them in SN1 and E1 reactions.
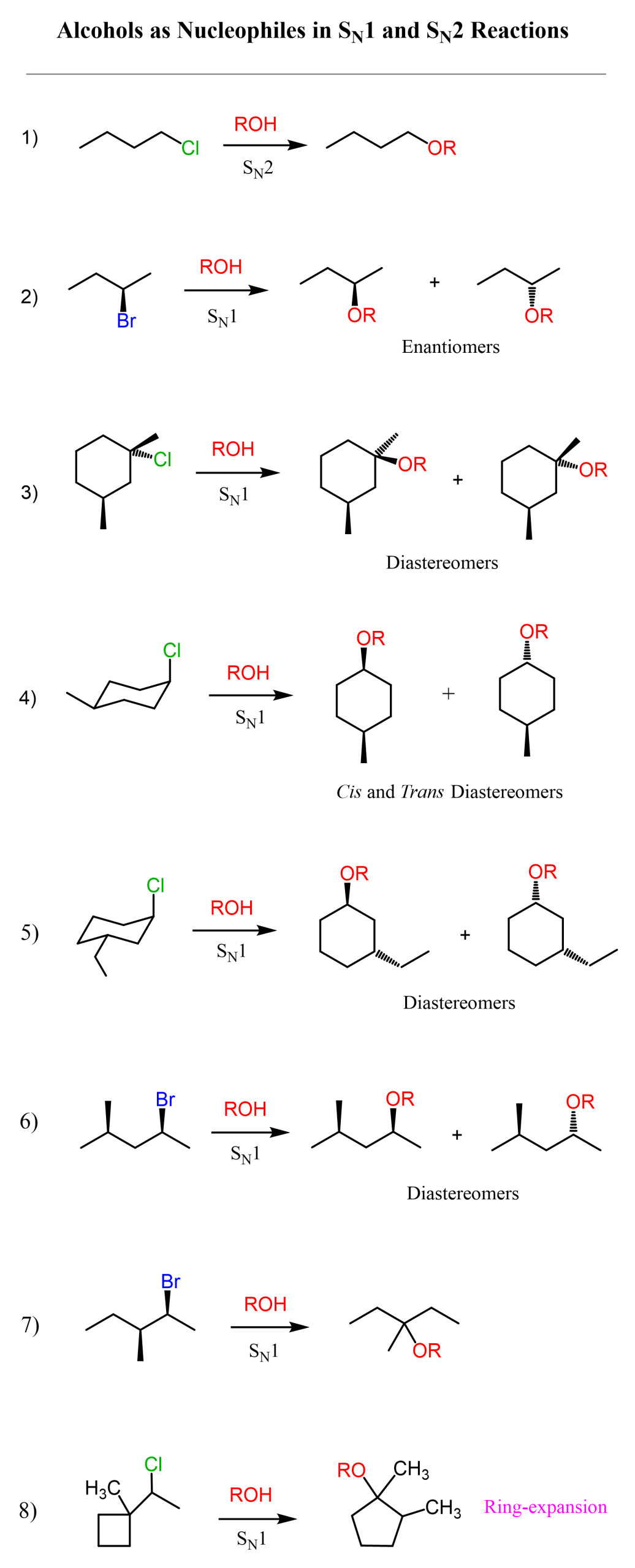
A primary alkyl halide will still react with an alcohol because of he great excess when used as a solvent; however, these reactions are slow and inefficient.
I have picked a variety of alkyl halides to demonstrate the stereochemistry of SN1 reactions. For chiral secondary alkyl halides, the typical outcome is a racemization of the chiral center. However, if another chiral center is present in the molecule that is not part of the reaction, a mixture of diastereomers is formed.
Check the articles on the stereochemistry of SN1 reactions as well as the conversion of alcohols to alkyl halides for more examples and practice problems.
On a side note, recall that SN1 and E1 reactions go hand in hand, and a mixture of substitution and elimination reactions is obtained. The use of heat most often favors the E1 path, and it should be the hint for you on the test when deciding between SN1 and E1 reactions.

Check this article for a detailed discussion on deciding between SN1 and E1 reactions.
Converting Alcohols into Good Nucleophiles
The alcohol is a neutral molecule, but if we deprotonate it into its conjugate base, the alkoxide ion, it becomes a good nucleophile and a strong base.

Having a good nucleophile/strong base excludes the possibility of SN1 and E1 reactions, so depending on the alkyl halide and the alcohol, the alkoxide can only participate in SN2 and E2 reactions.
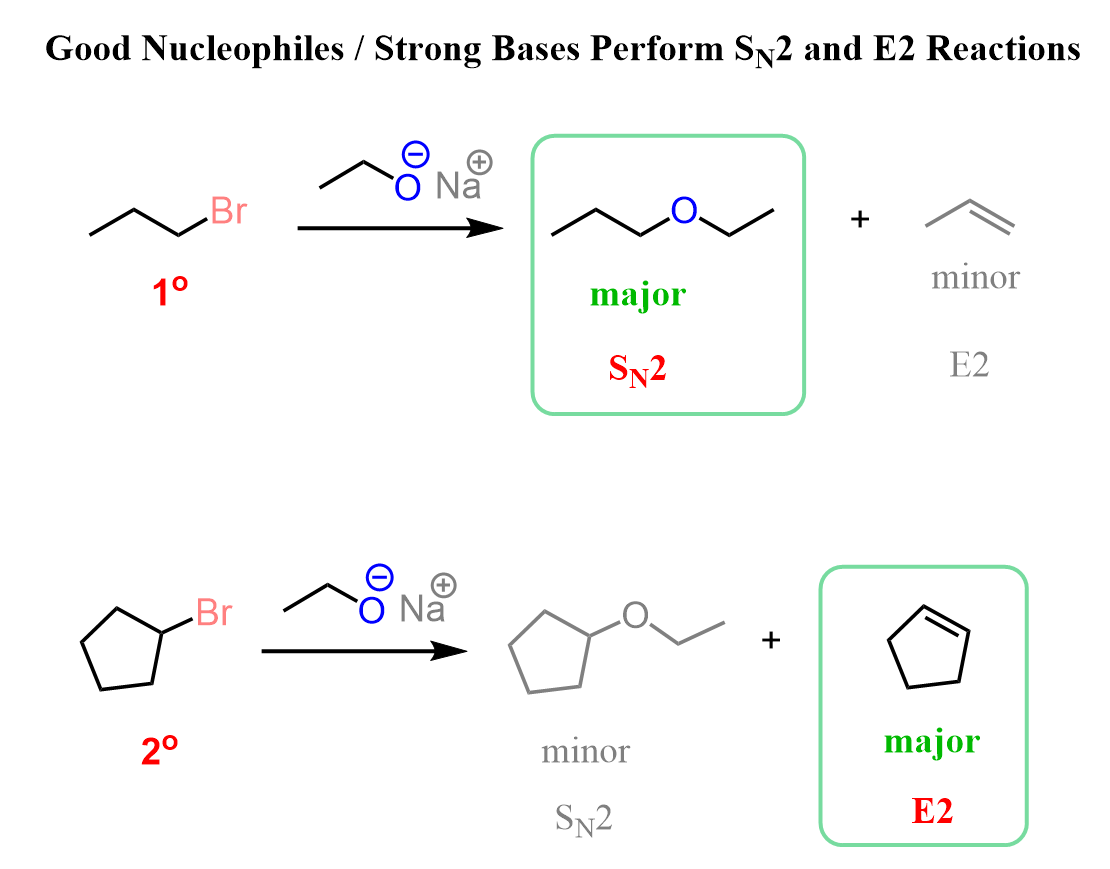
Notice that with primary alkyl halides, the SN2 mechanism predominates, while with secondary and especially tertiary ones, E2 is the main path of these reactions. The SN2 reaction of alkoxide ions with alkyl halides, forming ethers, is known as the Williamson ether synthesis, so feel free to check this post for more such conversions.
If a bulky alcohol, and consequently, its conjugate base, such as potassium tert-butoxide (tBuOK) is used, E2 elimination occurs regardless of whether we use a primary or secondary alkyl halide.
For example, 1-bromopropane is a primary alkyl halide, and it gives an SN2 product when reacted with an ethoxide ion (non-hindered strong base/nucleophile), whereas the reaction with tBuOK, a sterically hindered strong base, gives E2 elimination.
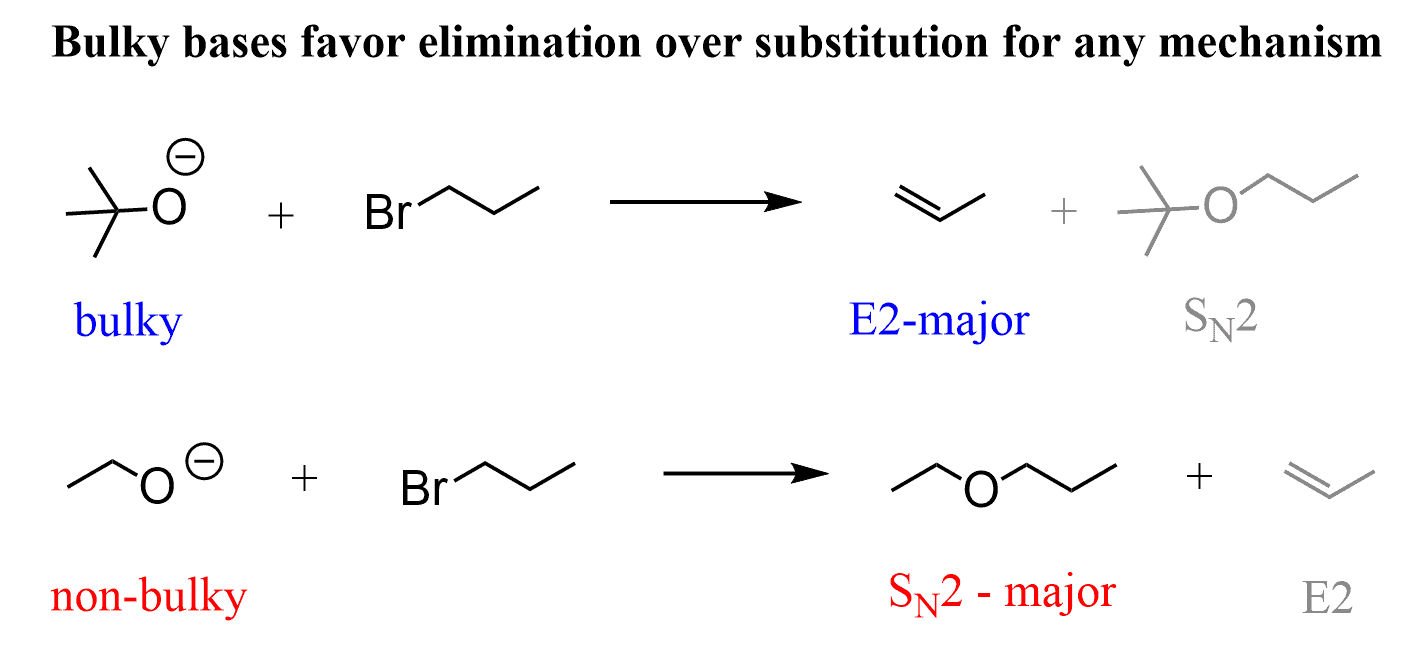
Check this article for more details on deciding between SN2 and E2 reactions.
Alcohols as Electrophilic Substrates
The simplest way of reacting with an alcohol in a substitution reaction is the use of hydrohalic acids HCl, HBr, and HI. The principle here is that the acid first protonates the OH group, making it a great leaving group (it is water), and the halide ion can now attack the carbon and expel water as the leaving group.
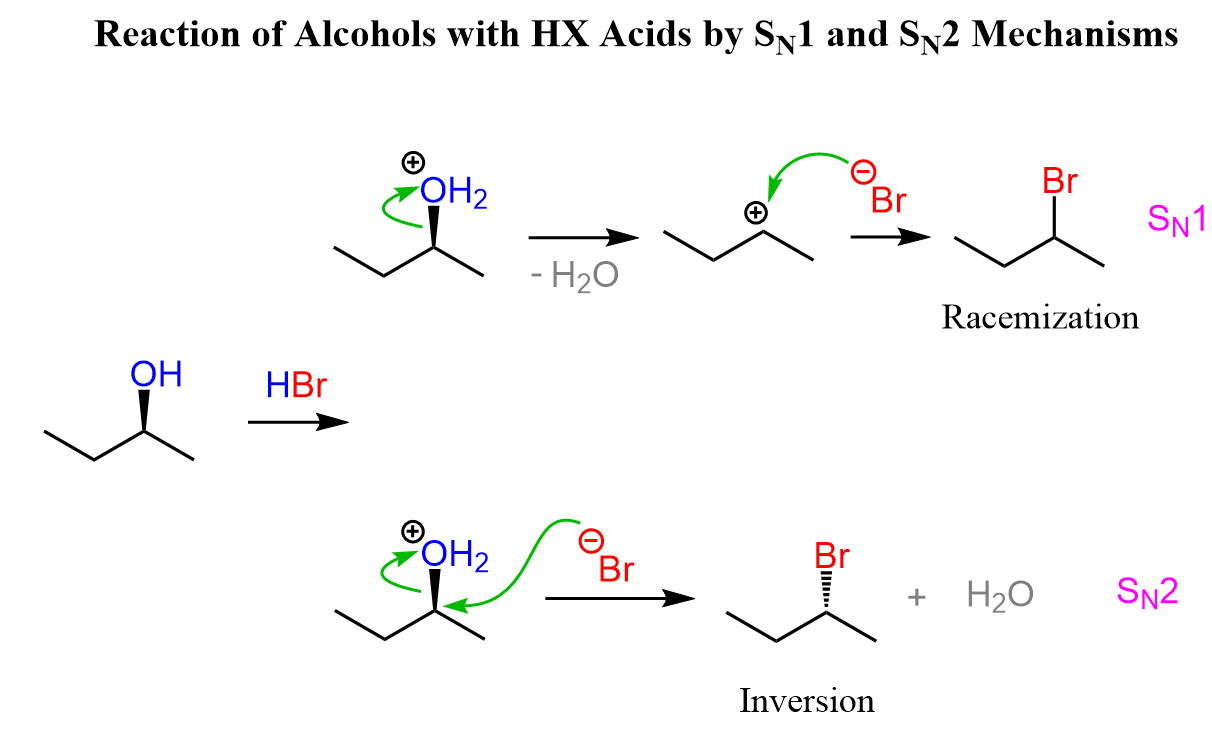
Notice that primary alcohols react by SN2, while tertiary alcohols can only participate in SN1 reactions. Both mechanisms are possible for secondary alcohols, and this is a disadvantage if we have a chiral alcohol, as the chirality is lost in the SN1 product.
Check also the articles on the stereochemistry of SN1 reactions as well as the conversion of alcohols to alkyl halides for more examples and practice problems.
Rearrangements in SN1 and SN2 Reactions of Alcohols
Another issue when using HX acids for the conversion of secondary and primary alcohols to alkyl halides is the possibility of a rearrangement because the reaction goes through a carbocation intermediate.
For example, a secondary alcohol may end up giving a tertiary alkyl halide when reacted with HBr or any other hydrogen halide.
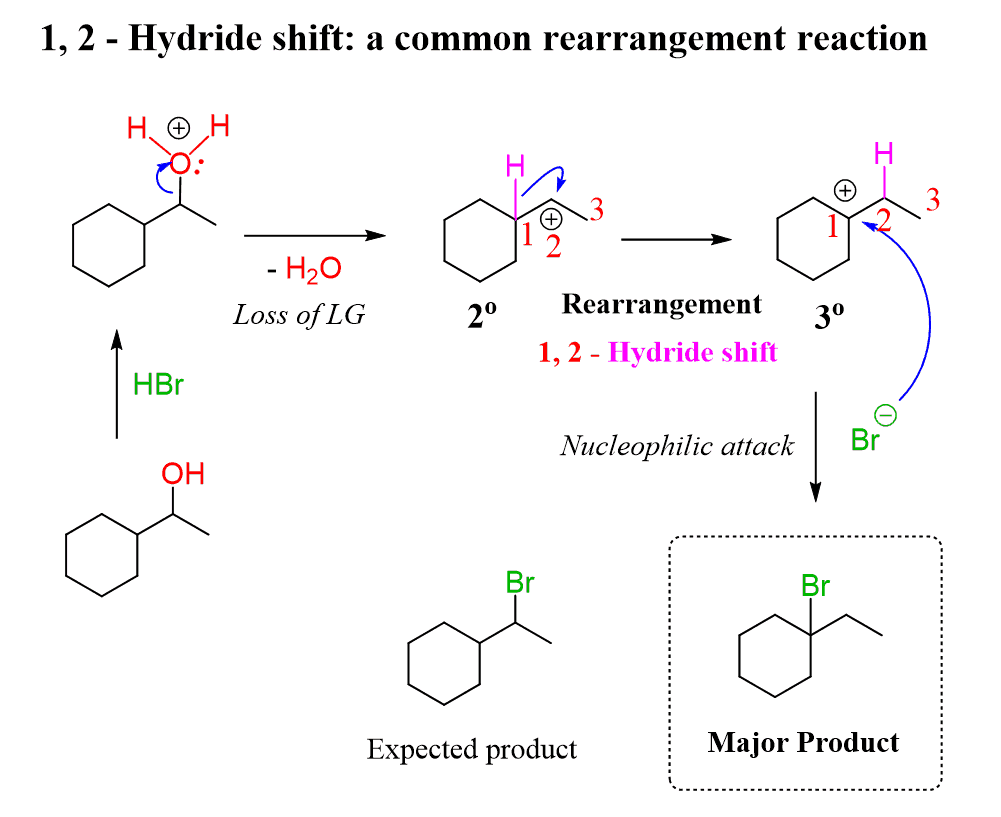
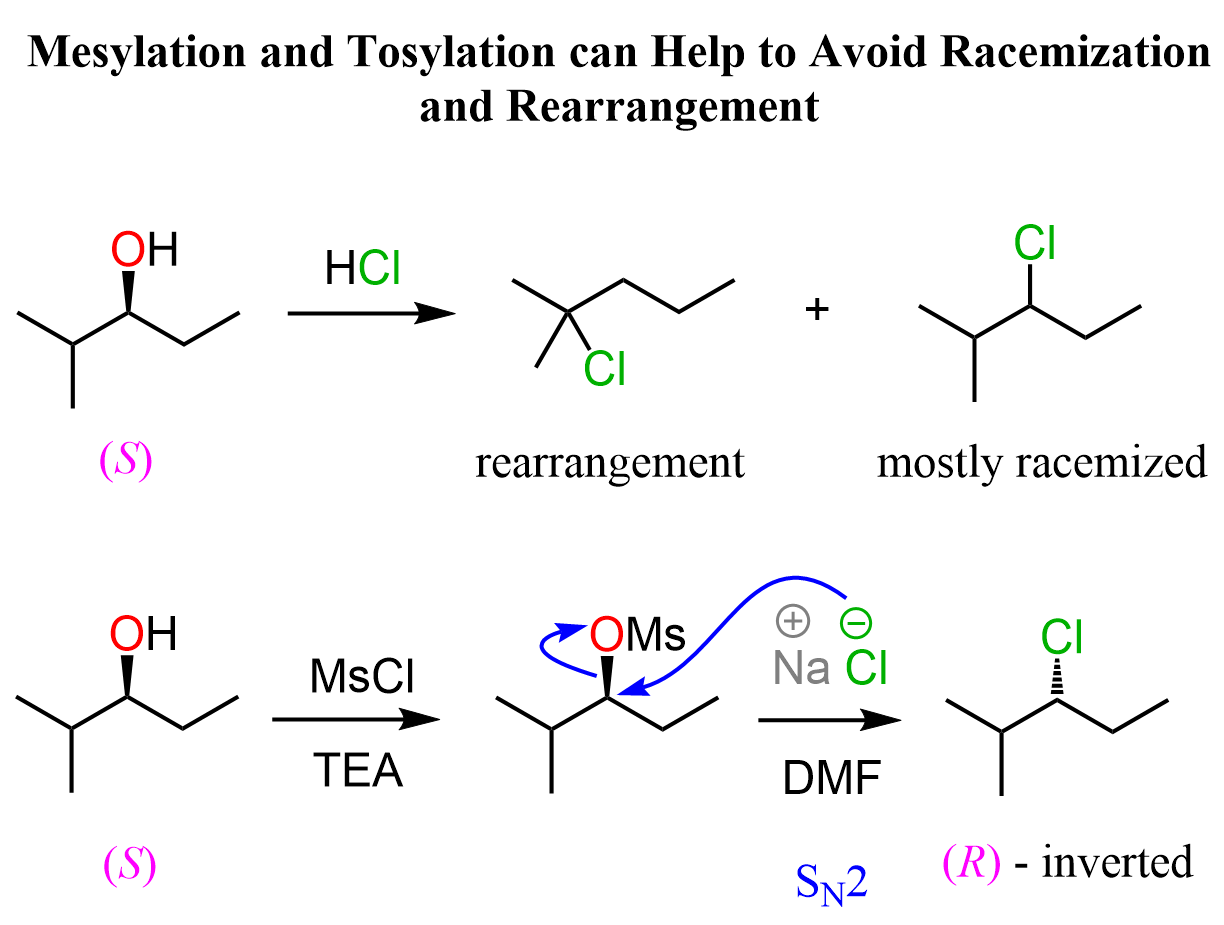
Like we did for avoiding the formation of carbocations to retain the control over stereochemistry, here as well, you can convert the alcohol to a mesylate or a tosylate and then use a good nucleophile in a polar aprotic solvent such as DMSO, DMF, or acetone to facilitate an SN2 substitution.
Ring Expansion Rearrangements of Alcohols
Ring-expansion rearrangements can also happen when alcohols with ring systems are reacted with HX acids. In this case, the carbocation intermediate is stabilized via an alkyl shift that forms a larger, thus a more stable ring. This typically happens with four or five-membered rings as they are rearranged to more stable 5- and 6-membered rings.
For example, reacting a secondary alcohol with an HX acid may lead to a ring expansion rearrangement where a less stable secondary carbocation is transformed into a more stable tertiary one. In the process, the four-membered ring becomes a five-membered ring, which is more stable due to reduced ring strain.
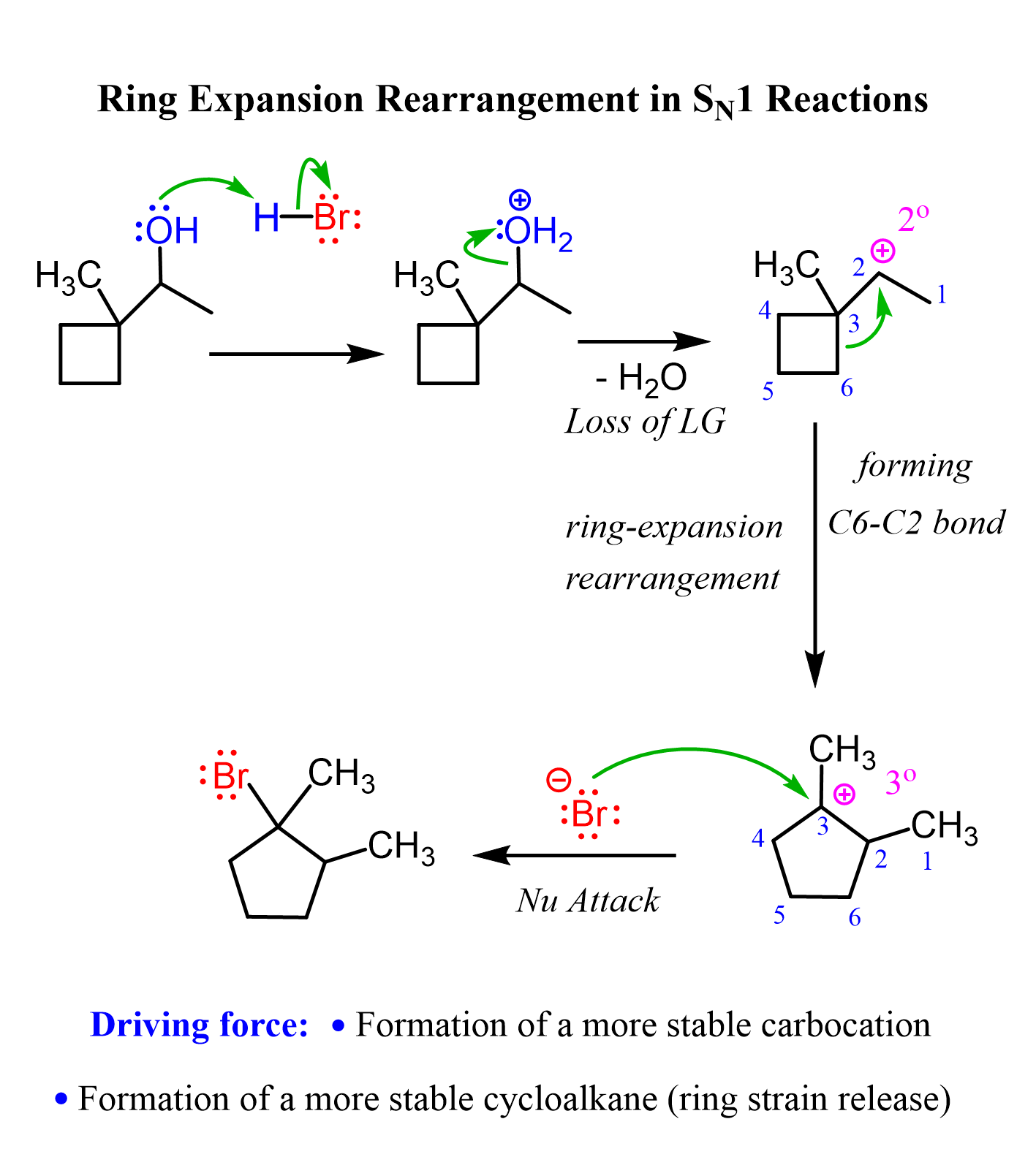
We have lots of examples, mechanisms, and practice problems on ring-expansion rearrangements, so be sure to check them out as well.
Mesylates and Tosylates for Converting OH into a Good Leaving Group
A common strategy for converting the hydroxyl group (–OH) into a good leaving group is using reagents like mesyl chloride (MsCl) or tosyl chloride (TsCl). The first part is the reaction of the alcohol with these chlorides in the presence of a base such as pyridine. This forms mesylates (ROMS) or tosylates (ROTS), both of which are excellent leaving groups due to their resonance-stabilized sulfonate structure.
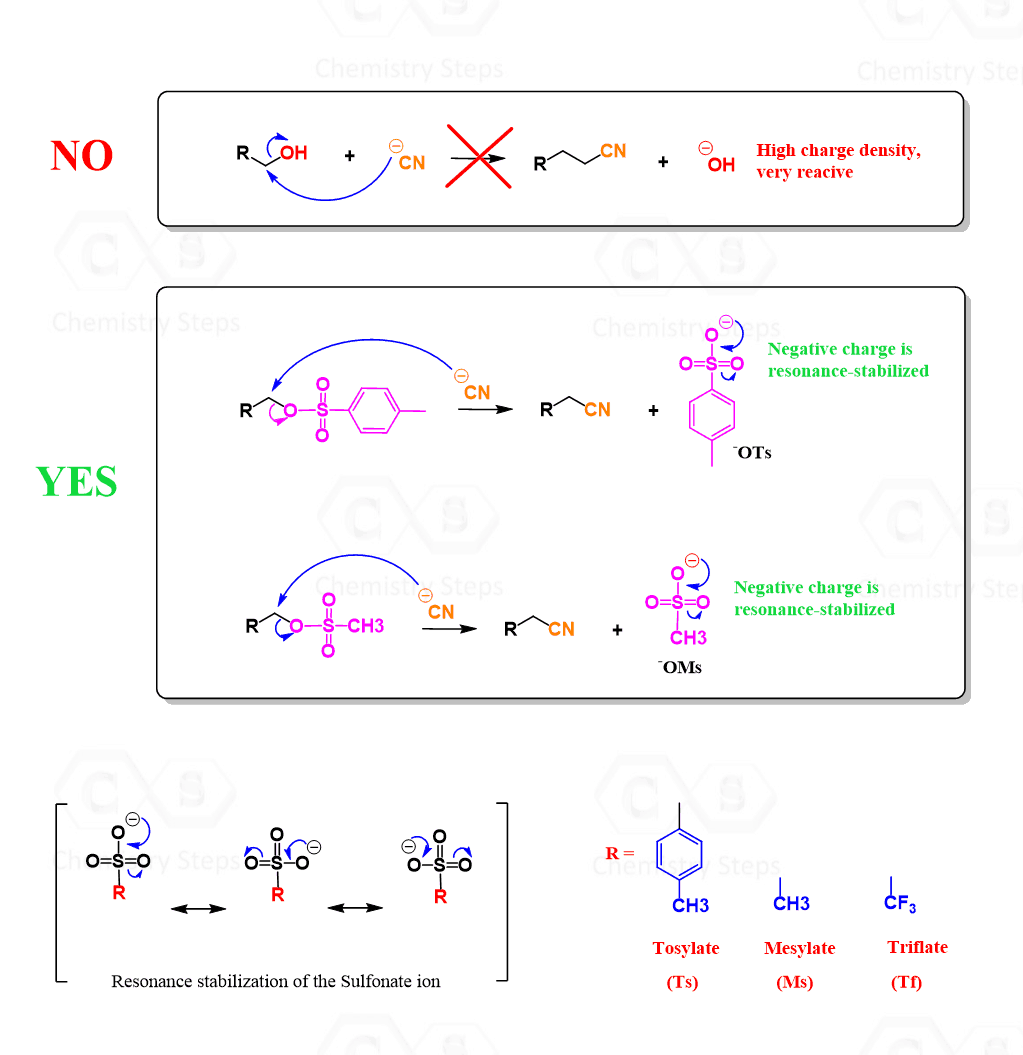
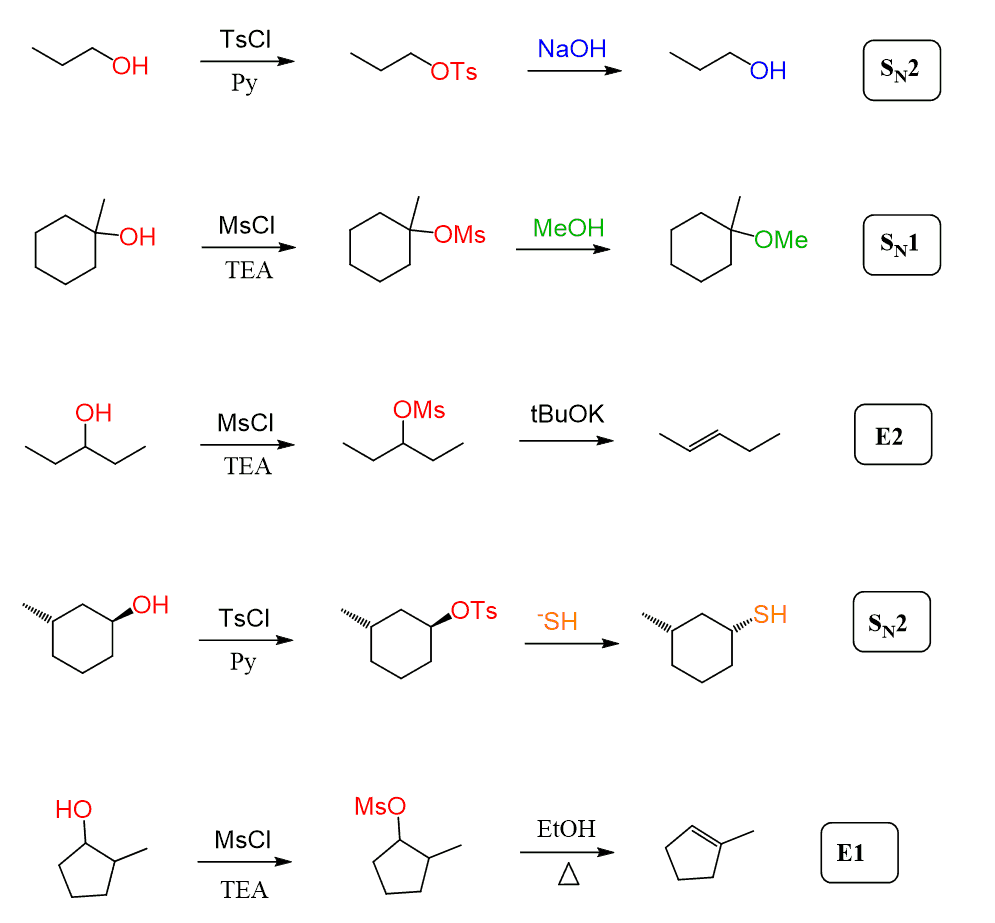
This transformation allows alcohols to participate readily in SN1, SN2, E1, and E2 reactions. Notice that this allows us to retain control over the stereochemistry, as, just like with alkyl halides, the SN2 conversion proceeds via inversion of configuration:
Here is also an example where mesylation and tosylation of alcohols can help react them by the SN2 mechanism and avoid undesired rearrangements that happen in acidic conditions:
Check this article for the mechanism of the formation of mesylates and tosylates, and this one for more strategies and details on converting alcohols to alkyl halides.
SOCl₂ and PBr₃ for Conversion of Alcohols to Alkyl Halides
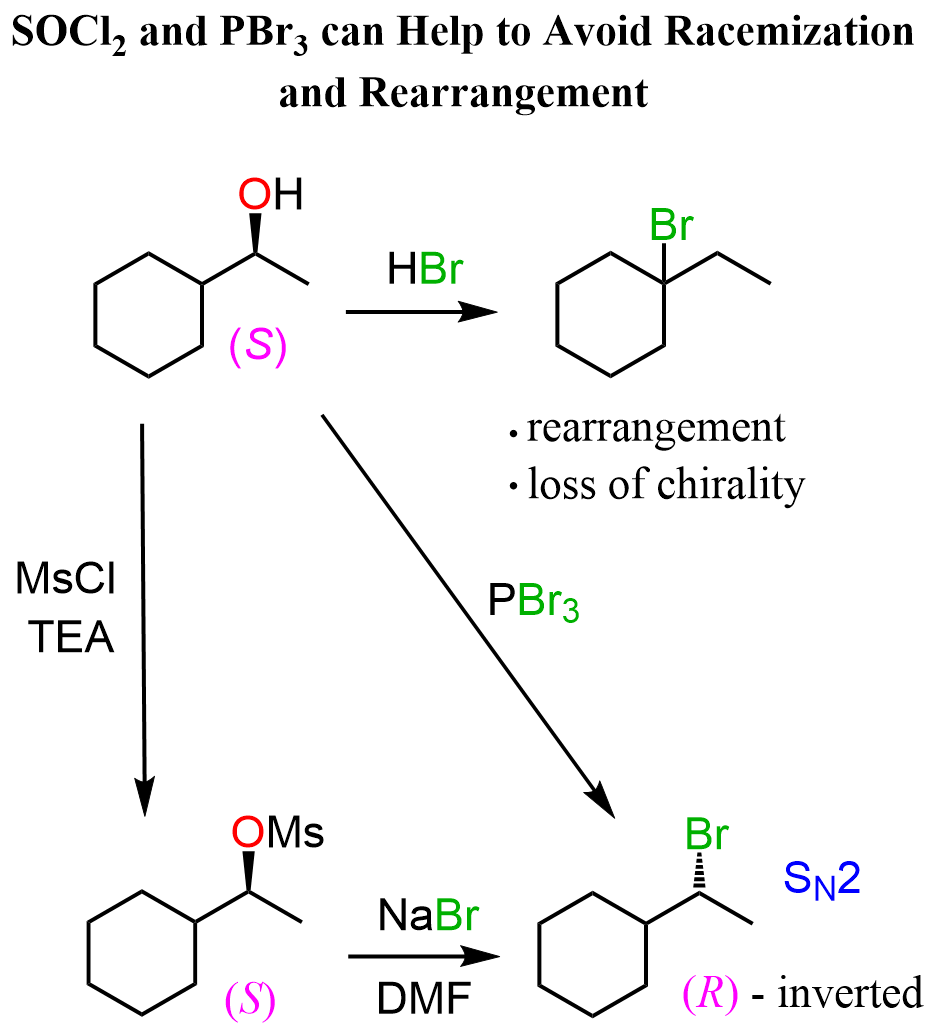
The use of SOCl₂ or PBr₃ is another great way of converting chiral secondary alcohols to alkyl halides via SN2 substitution. This means we keep control over the stereochemistry without allowing the reaction to go through SN1 reactions, thus giving racemized chiral centers.
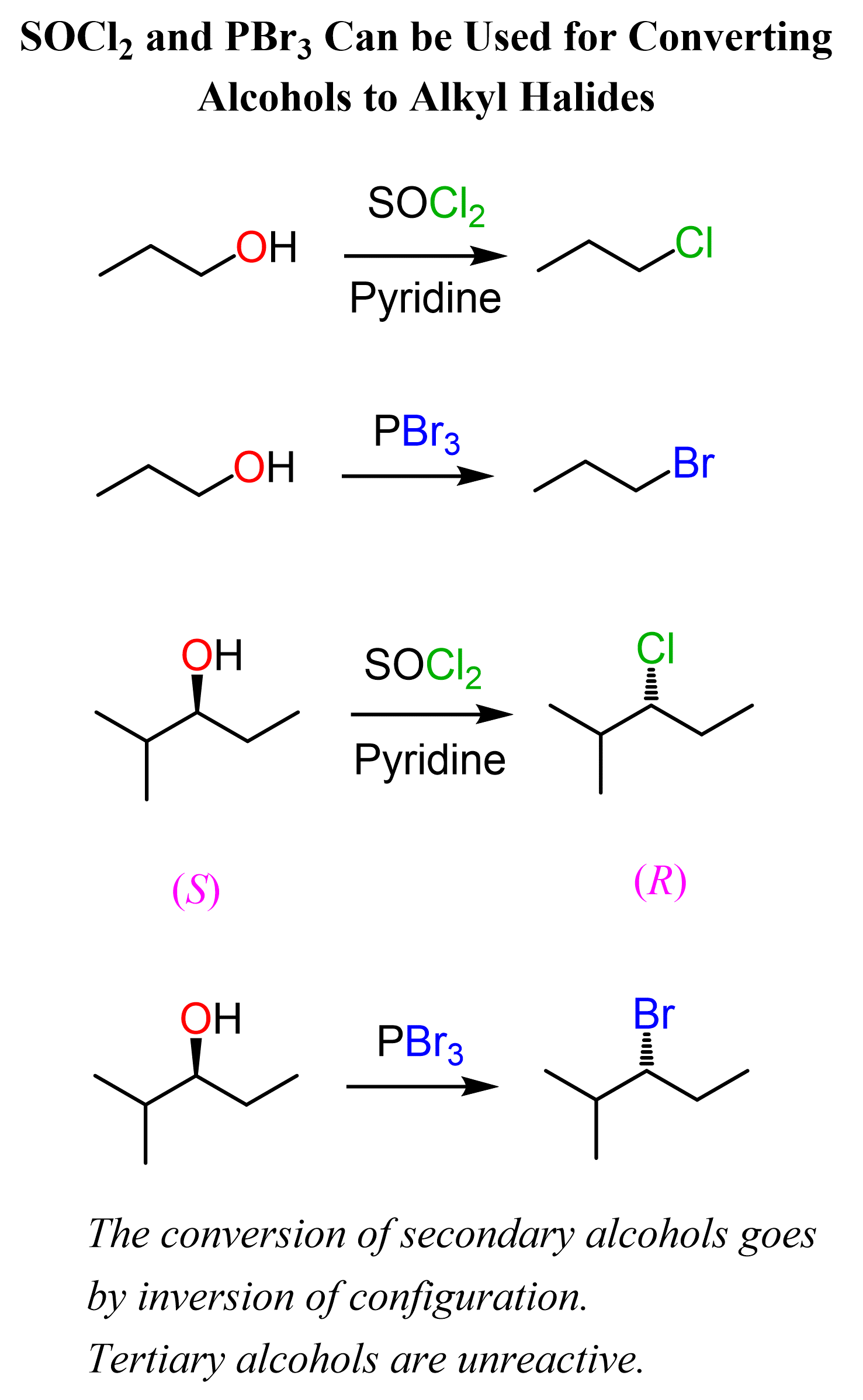
Once again, this is analogous to using mesylates and tosylates to avoid possible loss of chirality, and rearrangements when alcohols are reacted with HCl, HBr, and HI for converting them into alkyl halides:
One limitation of SOCl₂ and PBr₃ for converting alcohols to alkyl halides is that they don’t work with tertiary alcohols – no reaction occurs.
Summary: Alcohols in SN1 and SN2 Reactions
Alcohols can act as both nucleophiles and electrophilic substrates, but their direct participation in SN1 and SN2 reactions is limited. As nucleophiles, alcohols are weak due to their neutral –OH group.
- To turn an alcohol into a good nucleophile and base (E2), we convert them to alkoxides, which greatly enhances their reactivity, favoring SN2 or E2 pathways depending on the alkyl halide.
- To use alcohols as electrophiles, they must first be activated since –OH is a poor leaving group. This can be achieved by protonation (e.g., with HCl, HBr, or HI) or by converting the OH into better leaving groups such as mesylates, tosylates, or alkyl halides using reagents like SOCl₂ and PBr₃. These transformations allow alcohols to undergo substitution and elimination reactions efficiently while providing control over mechanism and stereochemistry.
- When using HX acids to convert secondary or primary alcohols, rearrangements can occur due to carbocation intermediates. These include hydride or alkyl shifts and ring expansion rearrangements that lead to more stable carbocations. To avoid this and retain stereochemical control, the alcohols are converted to a mesylate or tosylate, SOCl2 or PBr3, and reacted via SN2 in a polar aprotic.