There are several ways of converting alcohols to alkyl halides. The most common, and perhaps the one that covers nuances of SN1 and SN2 reactions is the use of HX acids (HCl, HBr, HI), so let’s start with that.
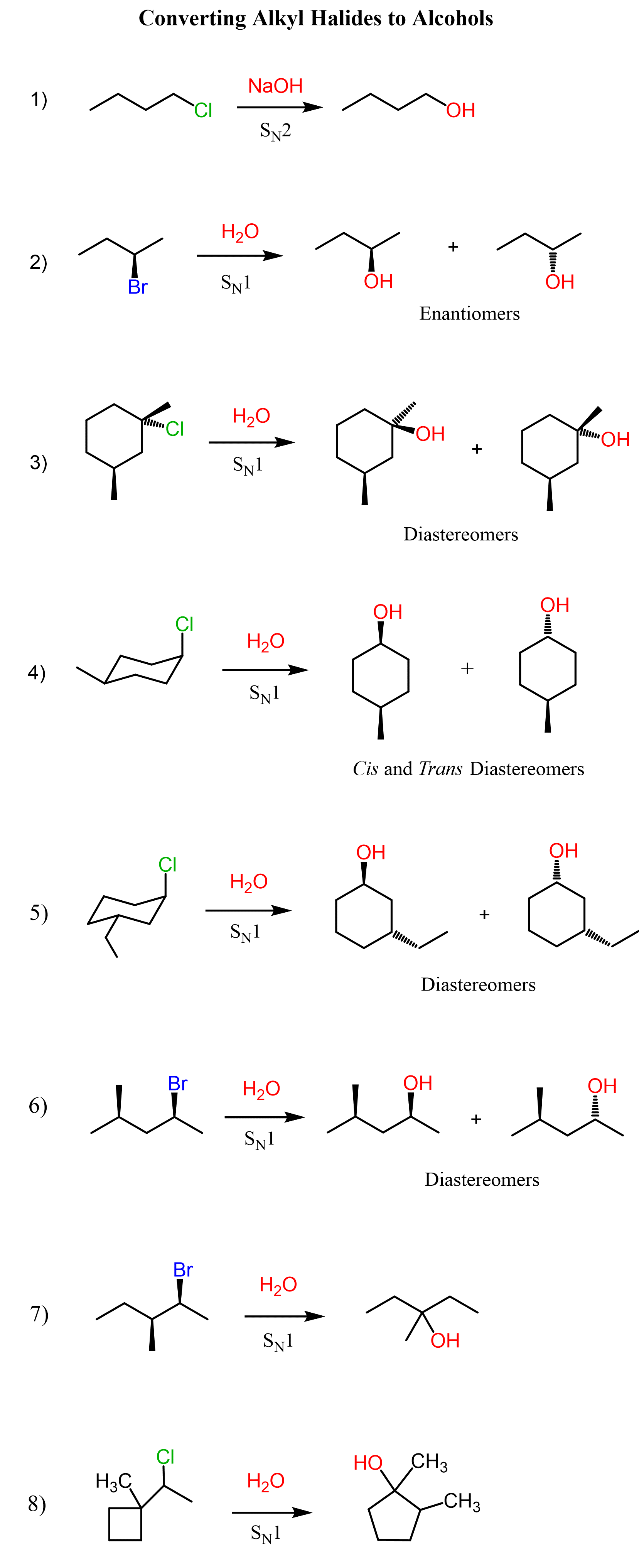
The conversion of an alcohol to an alkyl halide is a substitution reaction as we are replacing the OH group with a halogen. However, the first thing you need to remember here is that the OH by itself is never a leaving group, at least in organic chemistry 1.
This means there must be a process converting the OH into a good leaving group before the substitution can occur.
In the case of acids, the OH is converted into a good leaving group via protonation. When protonated, we now have water as a leaving group, which is obviously a very stable molecule and thus a great leaving group:
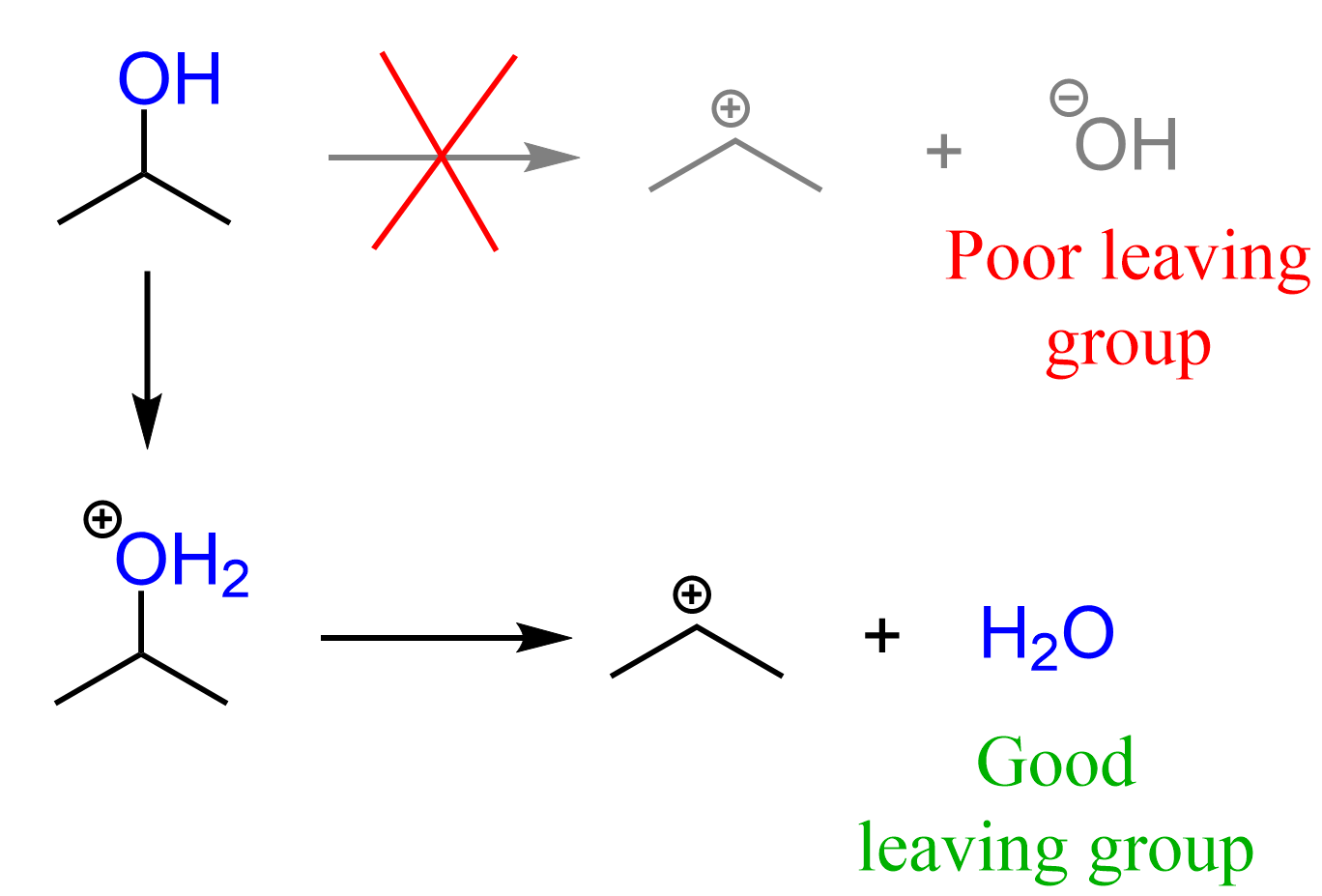
Now, whether water leaves on its own or is expelled by the halogen nucleophile depends on the structure of the alcohol. This, in turn, defines if the substitution mechanism is SN1 or SN2.
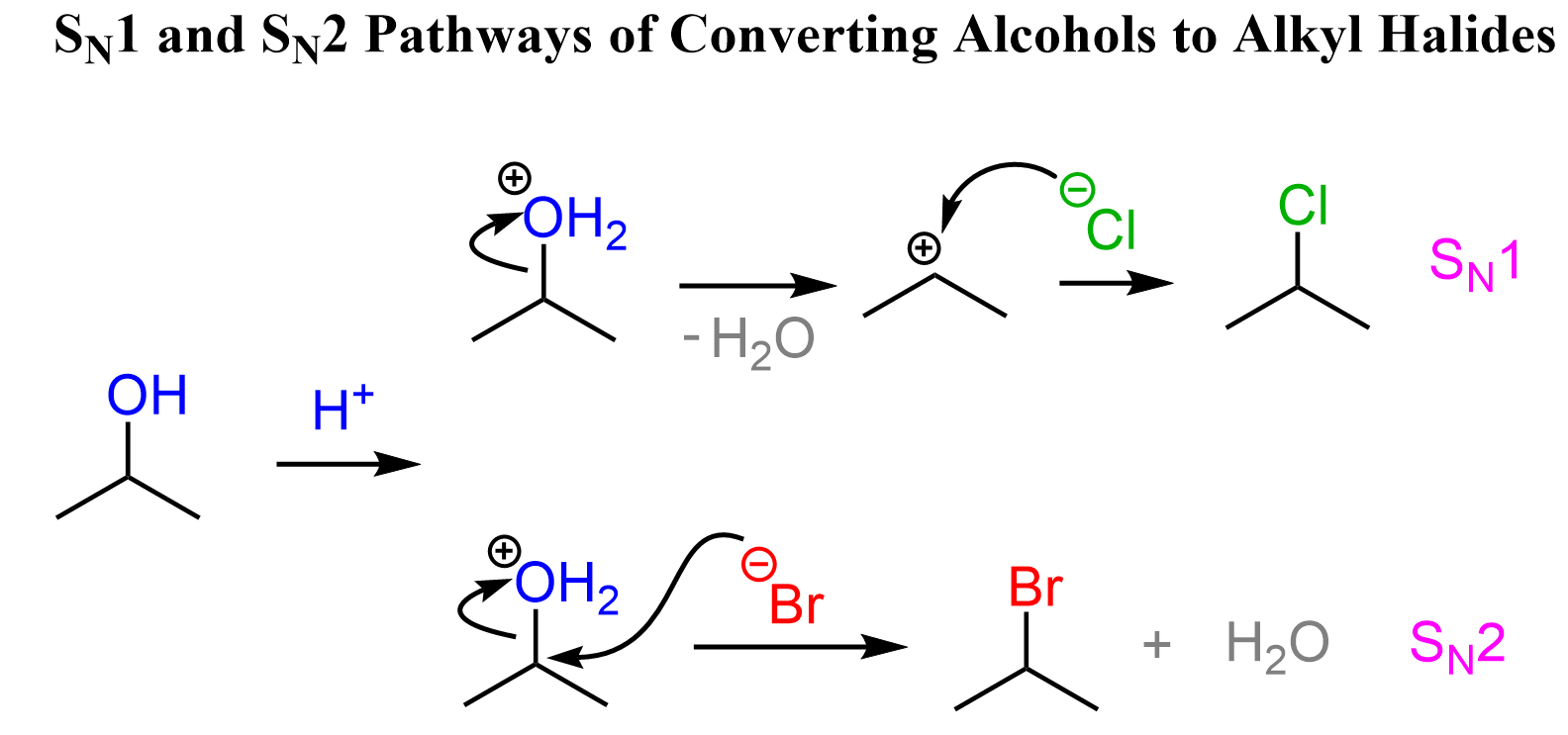
Primary Alcohols to Alkyl Halides
Methyl and primary alcohols undergo SN2 substitution when reacted with a hydrogen halide. The acid first protonates the OH group converting it to water leaving group which, in the next step, is kicked out by the halide ion:

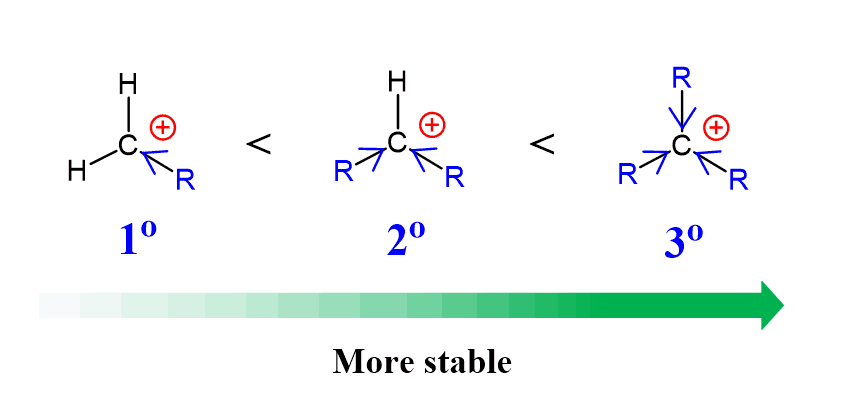
This is due to the low stability of methyl and primary carbocations, as well as the accessibility of the primary carbocation, as it is sterically unhindered. Remember, carbocations get more stable with the number of alkyl groups connected to the positively charged carbon atom. Therefore, methyl and primary substrates undergo SN2 rather than SN1 reactions.
Secondary Alcohols to Alkyl Halides
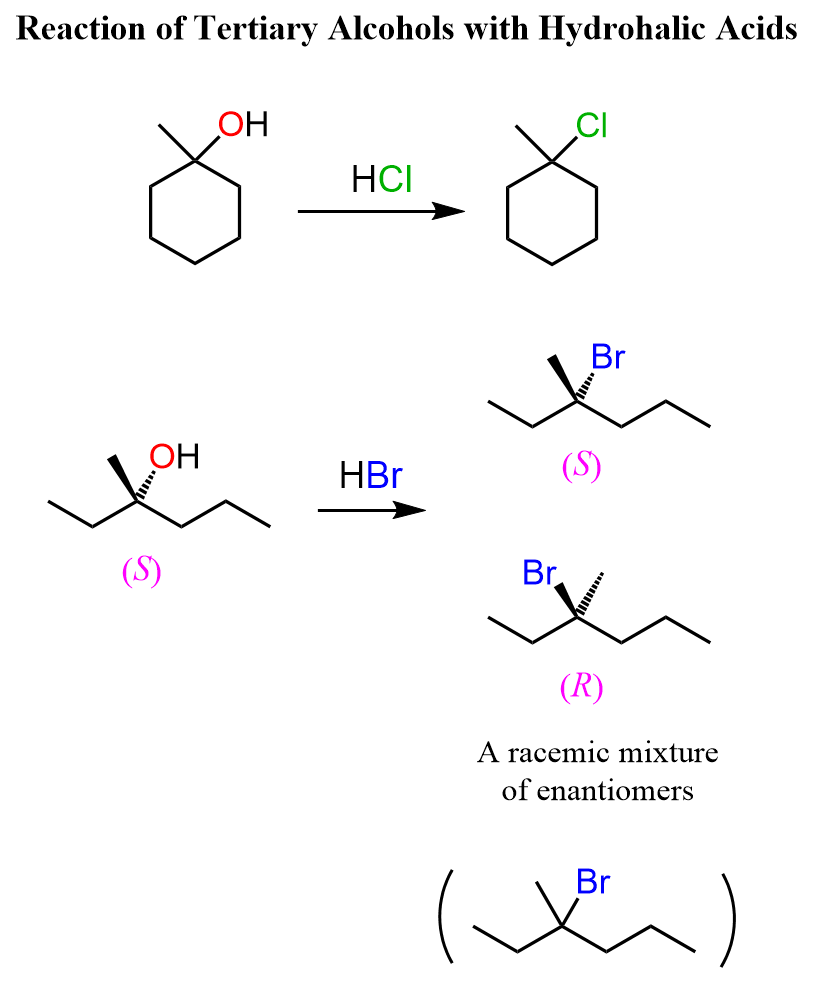
Secondary alcohols react by both SN2 and SN1 mechanisms, therefore, there is no good control over the stereochemical outcome of the reaction. In a conventional SN2 reaction, we know that the chirality center is inverted, therefore, we know what configuration of the product we’ll get.
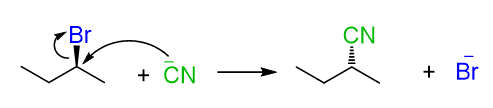
This is not the case when alcohols are reacted with acids because although the halide might be a good nucleophile, it is often an aqueous solution or a significant amount of water is present, which is a polar protic solvent and suppresses the reactivity of the nucleophile. Therefore, a chiral secondary alcohol will often give a racemic mixture of alkyl halides when reacted with an acid:

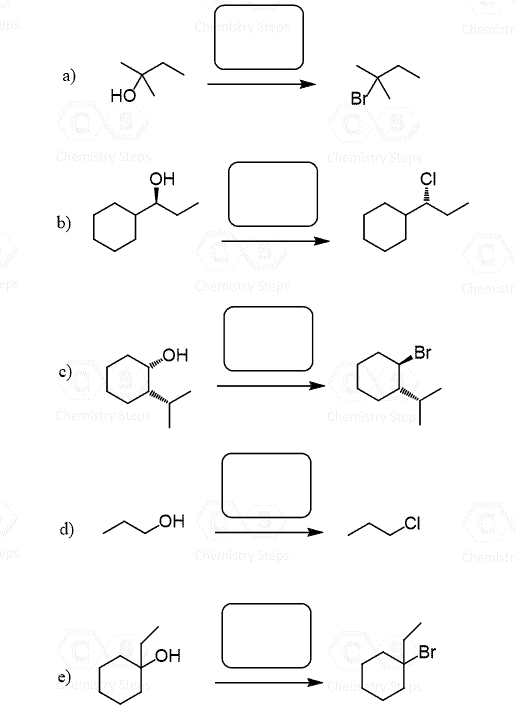
The stereochemistry of the product is very important, so a natural question here would be how to control it when converting chiral alcohols to alkyl halides. There are a couple of workarounds you need to know. One of them is the use of mesylates and tosylates which are essentially forms of the alcohol where the OH is converted into good leaving groups. Instead of the HX acids, mesylates, and tosylates are reacted with the corresponding halide ions in polar aprotic solvent to enforce the SN2 mechanism:
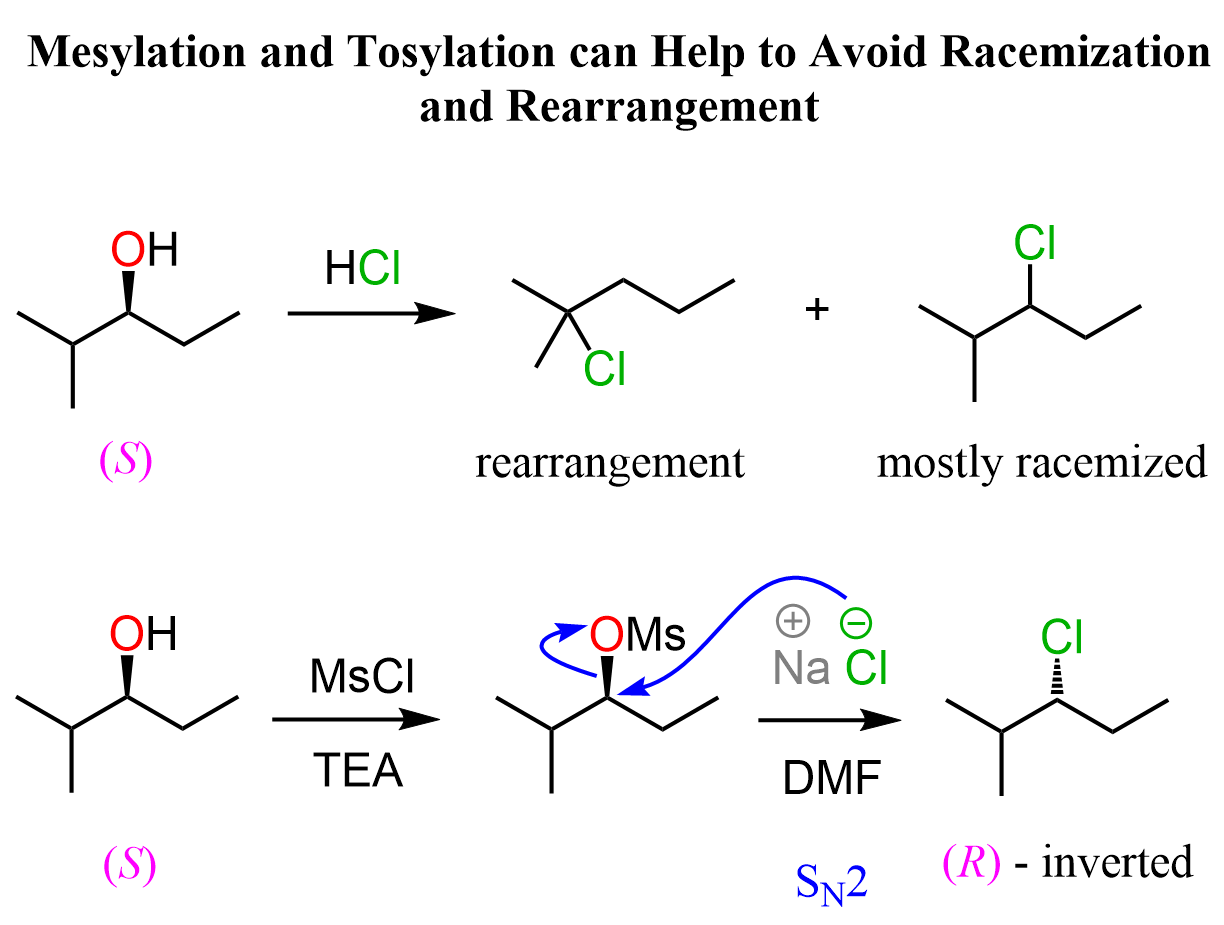
You can read more about the mesylates and tosylates in this article.
Another strategy to control the stereochemistry of alcohol conversion to alkyl halides is the use of SOCl2 and PBr3. Both of these can be used for converting primary and secondary alcohols to alkyl halides. Importantly, the absolute configuration of the chiral center is inverted as the substitution of the activated OH group by the halide ion goes by an SN2 mechanism:
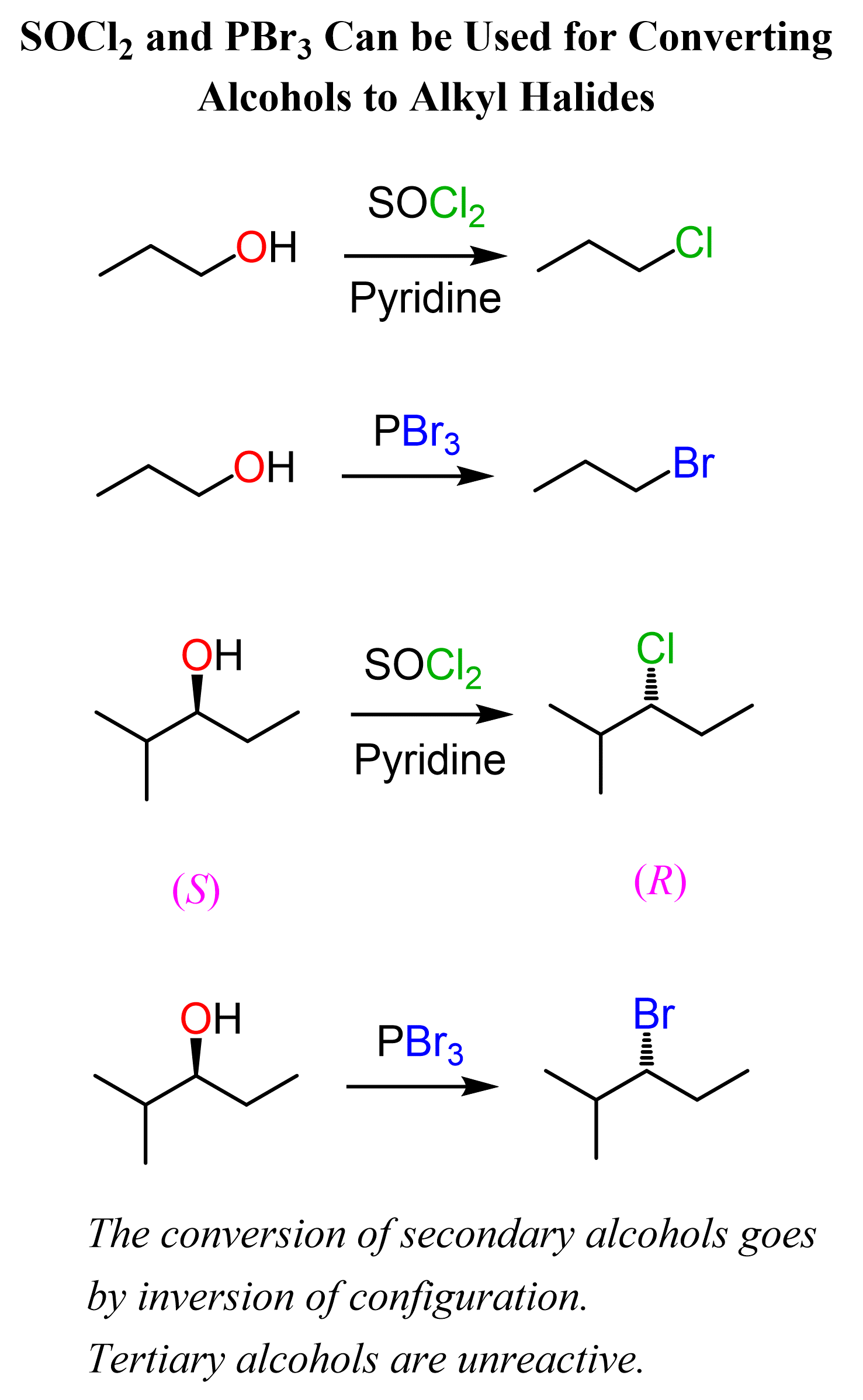
One drawback of SOCl2 and PBr3 is that they do not work with tertiary alcohols because of the steric hindrance. Therefore, tertiary alcohols are normally converted to alkyl halides by reacting them with HX acids. You can read more about the use of SOCl2 and PBr3 agents for the conversion of alcohols to alkyl halides here.
Rearrangements in Reactions of Alcohols with Acids
Another issue with the conversion of secondary and primary alcohols to alkyl halides is the possibility of a rearrangement because the reaction goes through a carbocation intermediate. For example, the following secondary alcohol gives a tertiary alkyl halide when reacted with HBr or any other hydrogen halide:
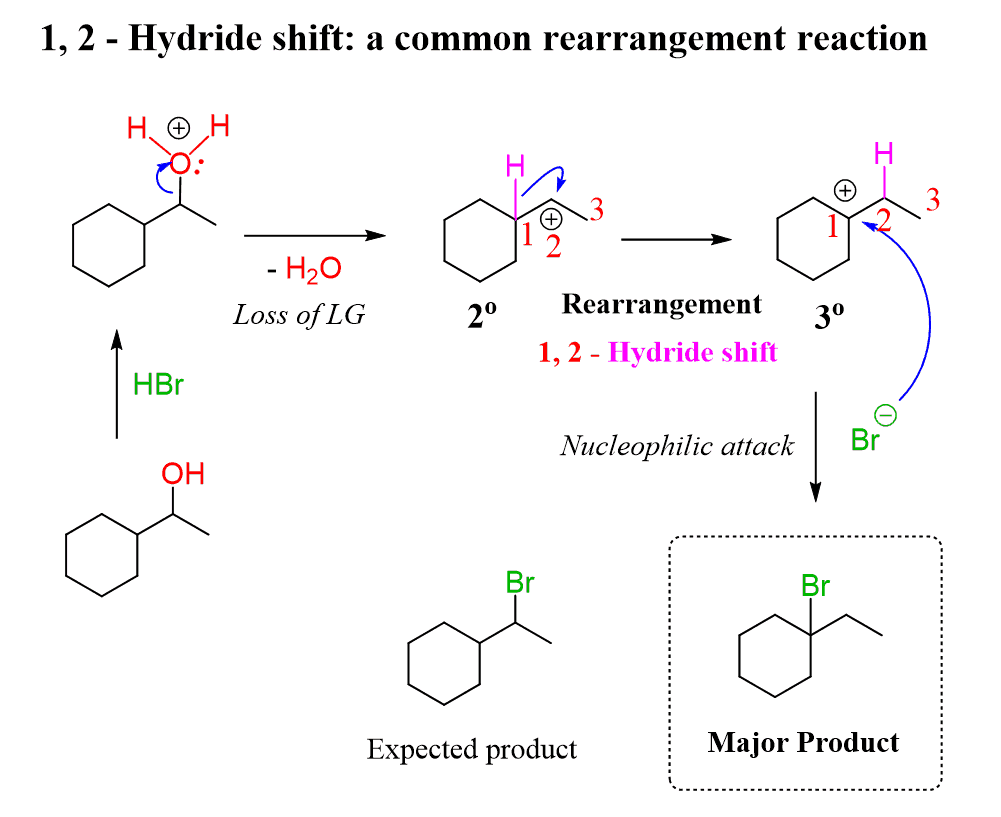
Once again, what you can do in these situations is convert the alcohol to a mesylate or a tosylate and react the latter with the salt of the given halogen. In this case, that would be NaBr in a polar aprotic solvent such as DMSO, DMF, acetone, etc..
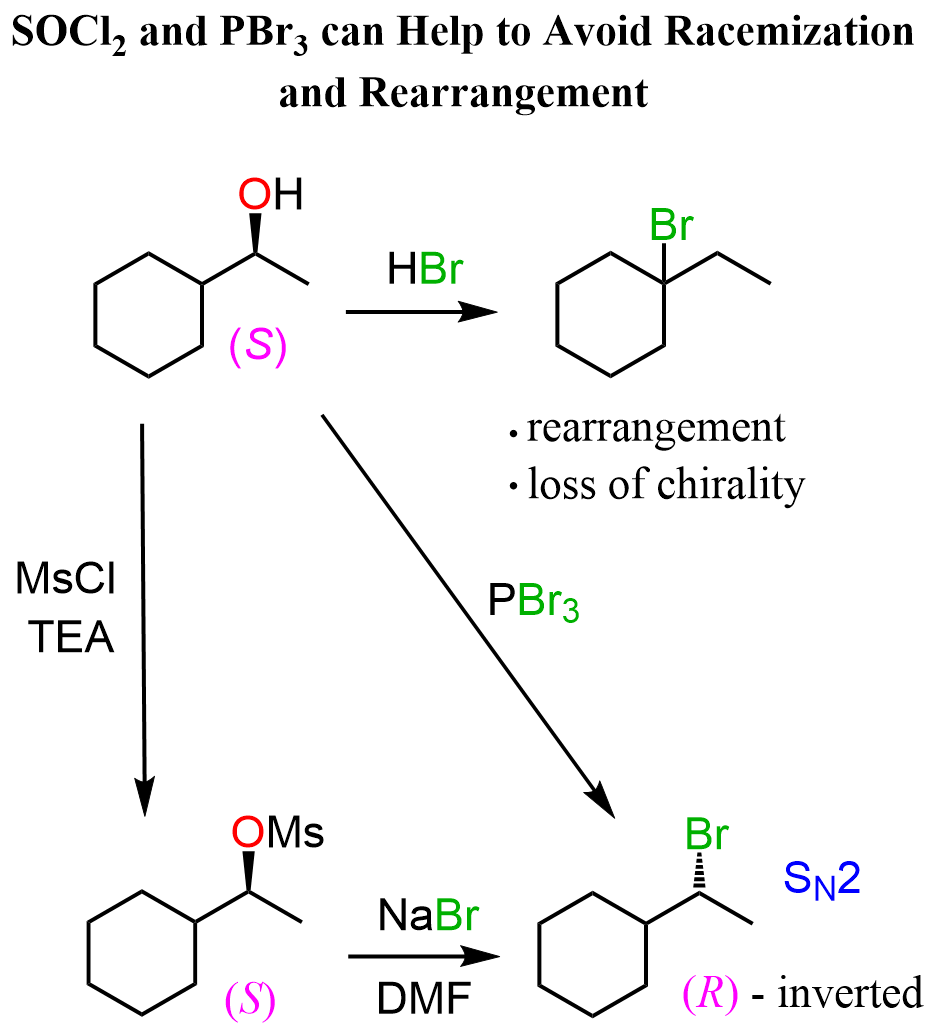
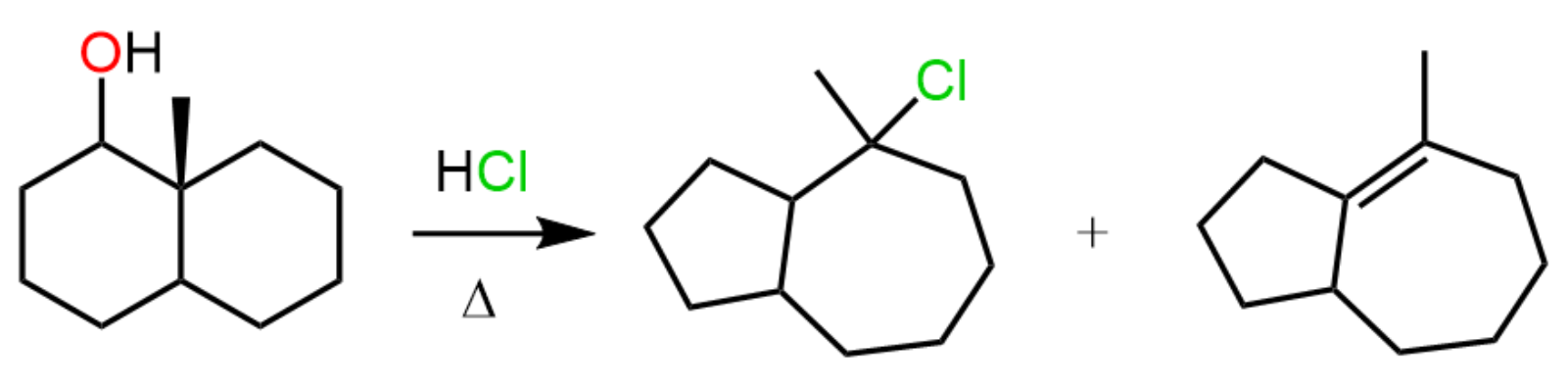
Ring Expansion Rearrangements
Ring expansion rearrangements occur in SN1 and E1 reactions when the carbocation intermediate can be stabilized via an alkyl shift, which is part of a four or five-membered ring. For example, the reaction of the following secondary alcohol with an HX acid proceeds via a ring expansion rearrangement because it converts the intermediate secondary carbocation to a more stable tertiary carbocation:
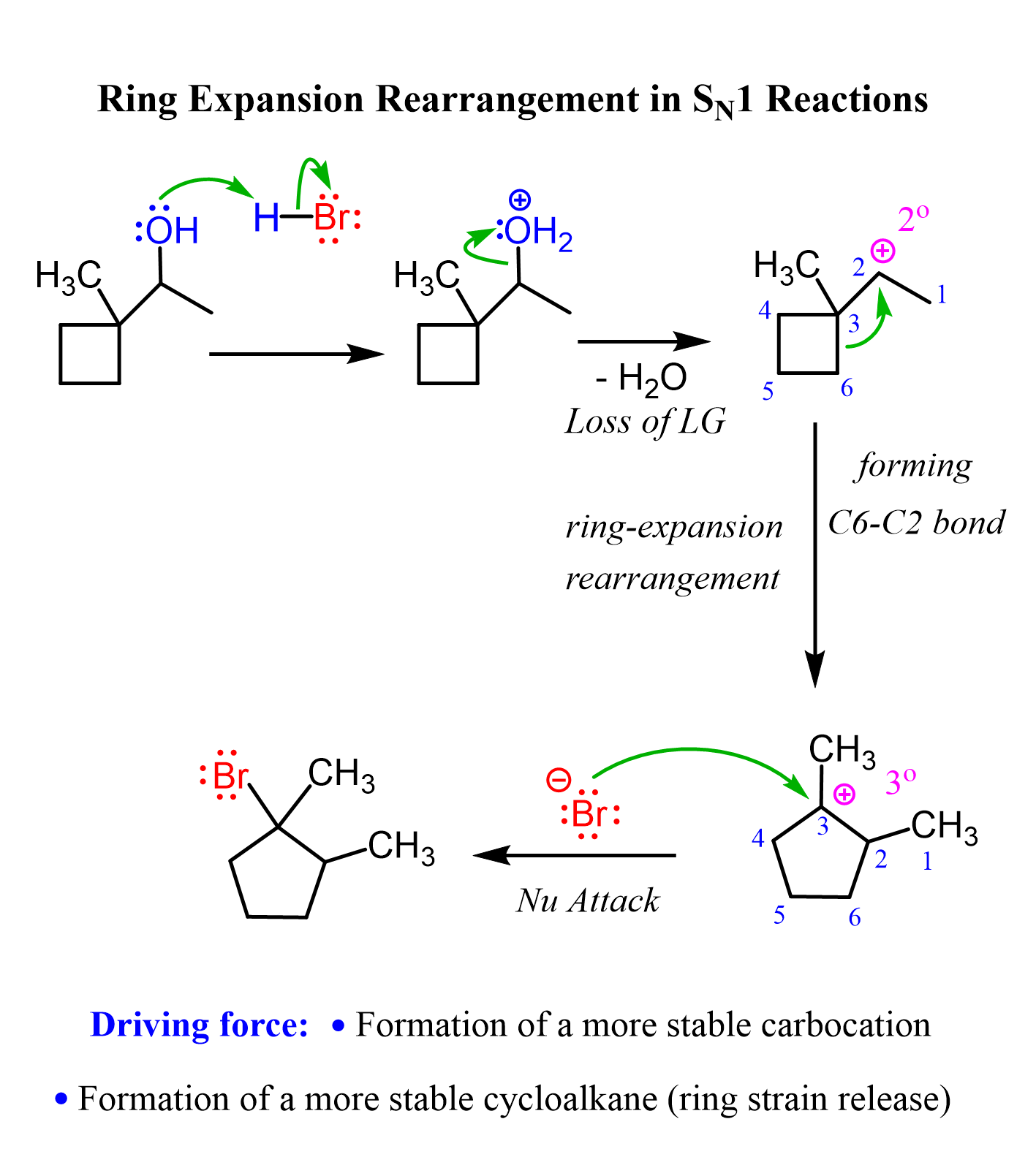
In the course of this carbocation stabilization, the four-membered ring is transformed into a five-membered ring, which is associated with less ring strain.
Tertiary Alcohols to Alkyl Halides
There are a couple of features of tertiary alcohols that make their conversion to alkyl halides more predictable. 1) They can only undergo SN1 substitution – no SN2, 2) Their intermediate tertiary carbocation is normally not prone to a rearrangement. This means we can react them with a hydrogen halide and prepare the corresponding tertiary alkyl halide, and if it is a chiral alcohol, we know that the chirality center will be racemized.
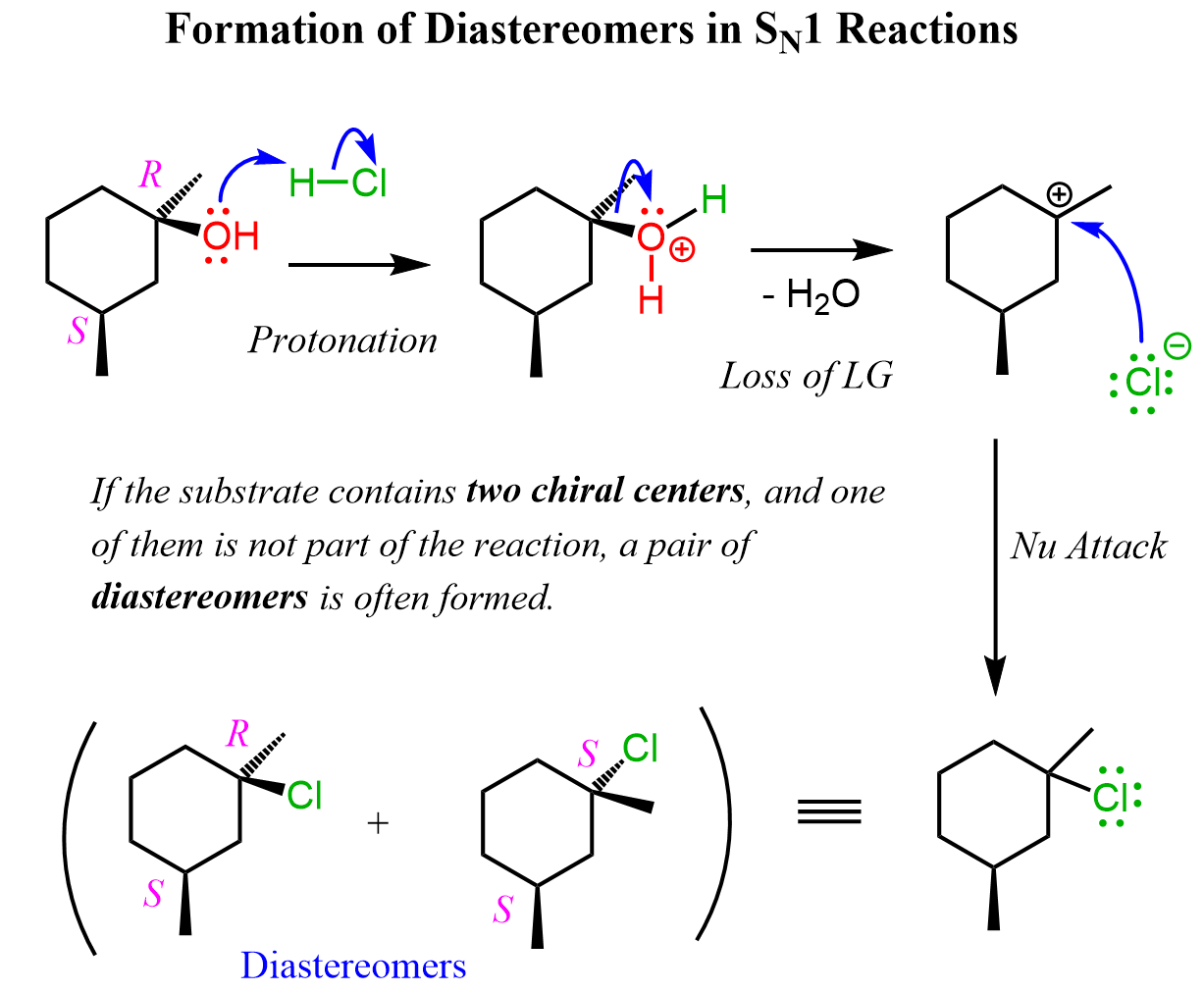
Diastereomeric Products in SN1 Reactions
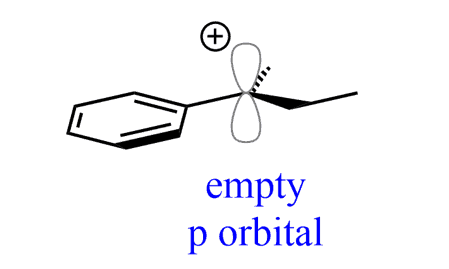
The most common outcome when a chiral substrate undergoes an SN1 reaction is the formation of a racemic mixture. This is because the carbocation intermediate is planar (the sp2 carbon), and it is attacked by the nucleophiles from both faces, thus both enantiomers are formed in equal amounts:
However, you also need to know that diastereomers can also be formed if the substrate has two or more chiral centers and only one of them is the reaction center. For example, the following tertiary alcohol has two chiral centers. One of them is racemized in the SN1 substitution reaction, whereas the second one remains intact over the course of the reaction; thus, a pair of diastereomers is formed:
We have a separate post on the stereochemistry of SN1 reactions, so feel free to check that out as well.
In the end, I wanted to mention that the reverse of these reactions, that is, the conversion of alkyl halides to alcohols, follows the same mechanistic and stereochemical patterns. Whether an alcohol is converted to an alkyl halide or vice versa depends on the concentration of the reagent. If we keep the alkyl halides, which we were talking about today, in a bottle of water, they will be converted back into alcohols provided proper temperatures. In the next article, we will discuss all the following conversions of alcohols to alkyl halides:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Converting Alkyl Halides to Alcohols
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- The Stereochemistry of SN1 Reactions
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions with Tons of Practice Problems
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz