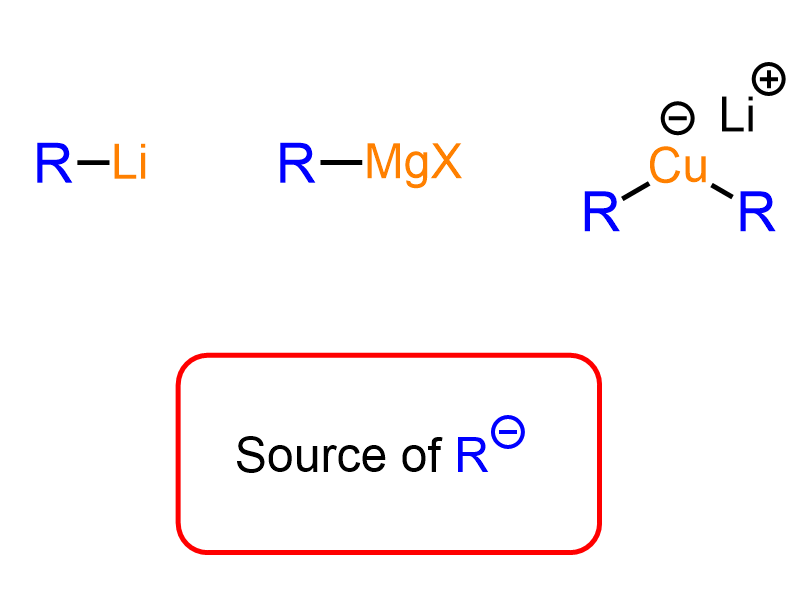
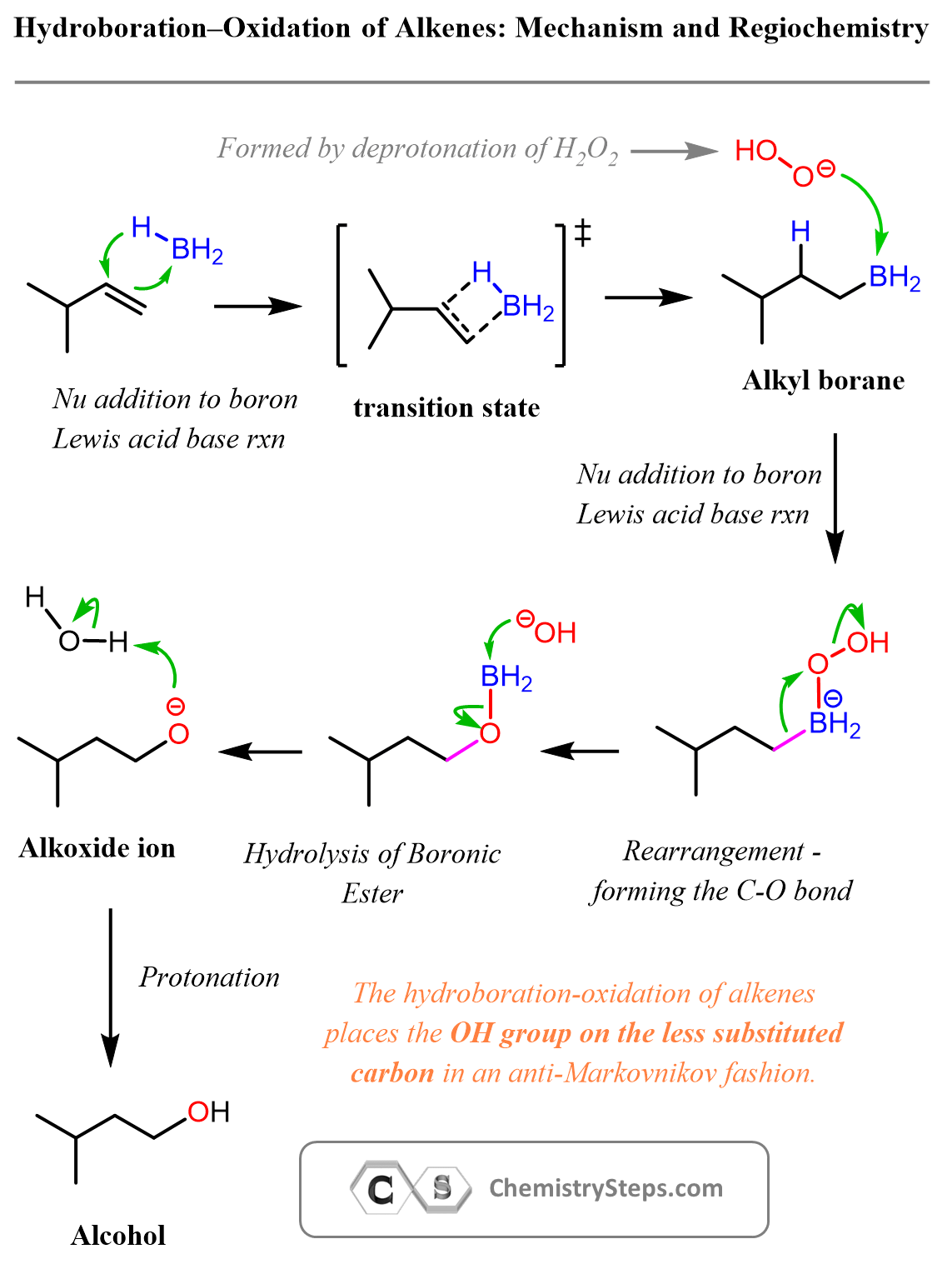
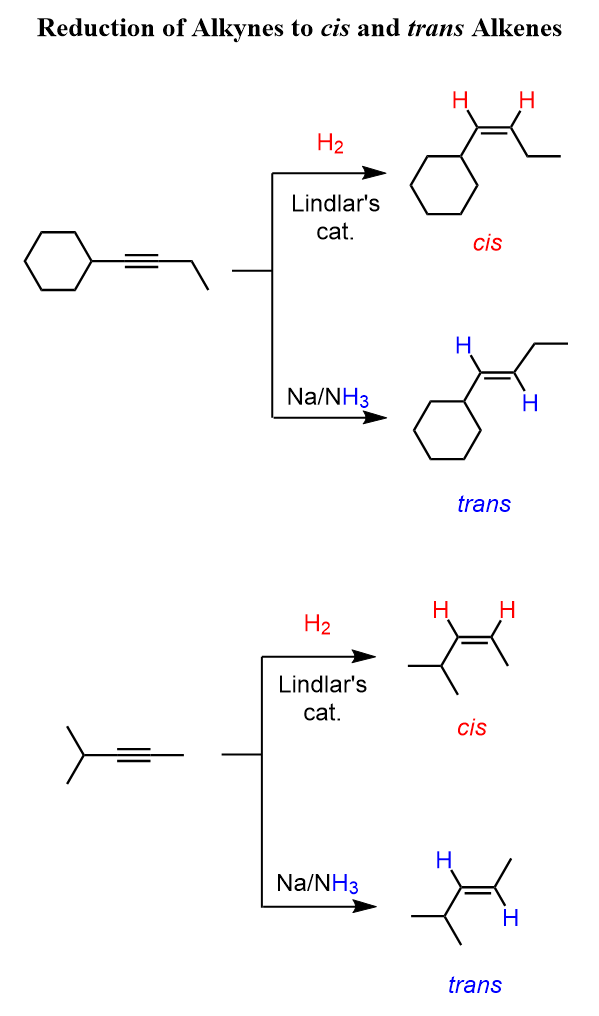
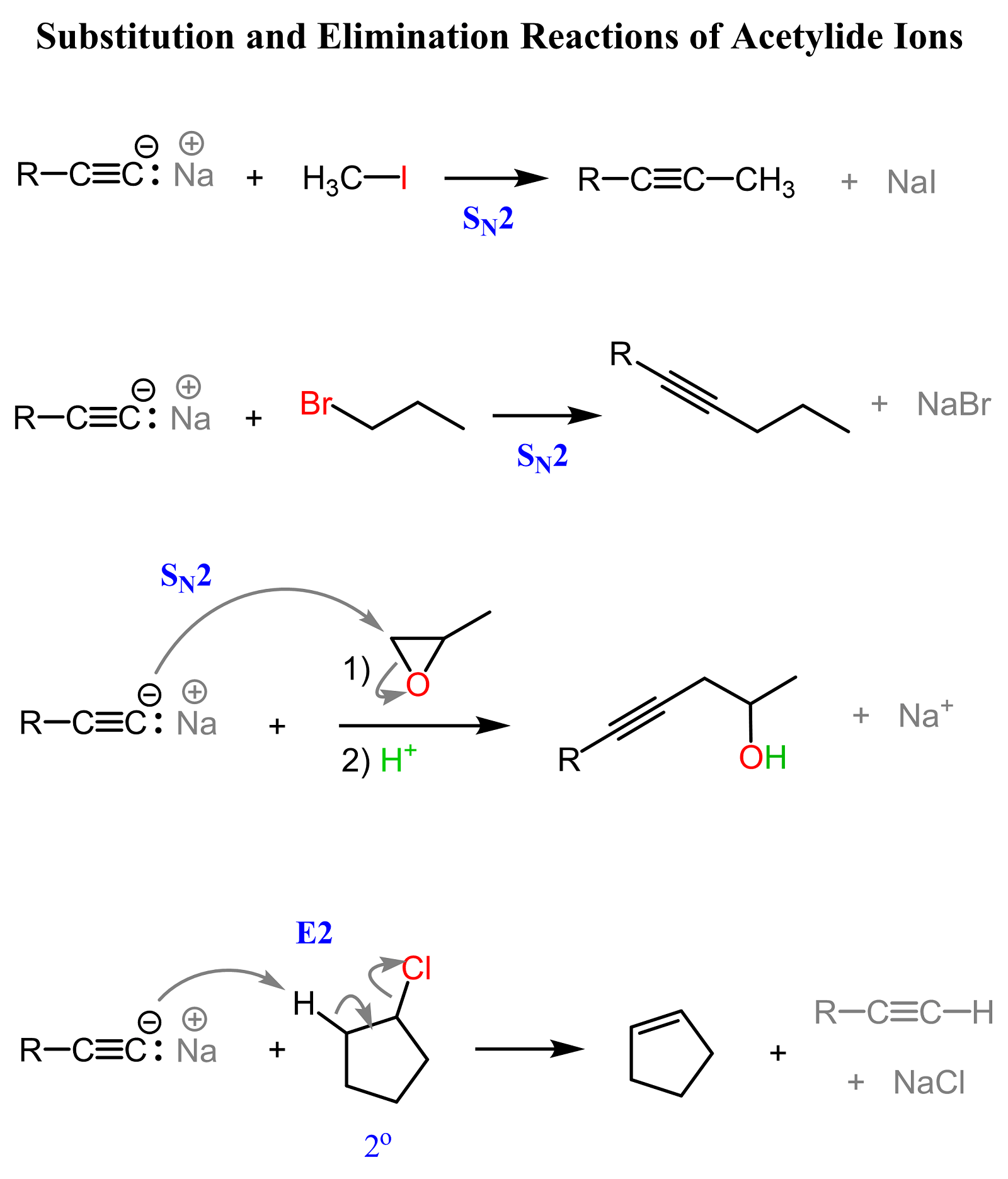
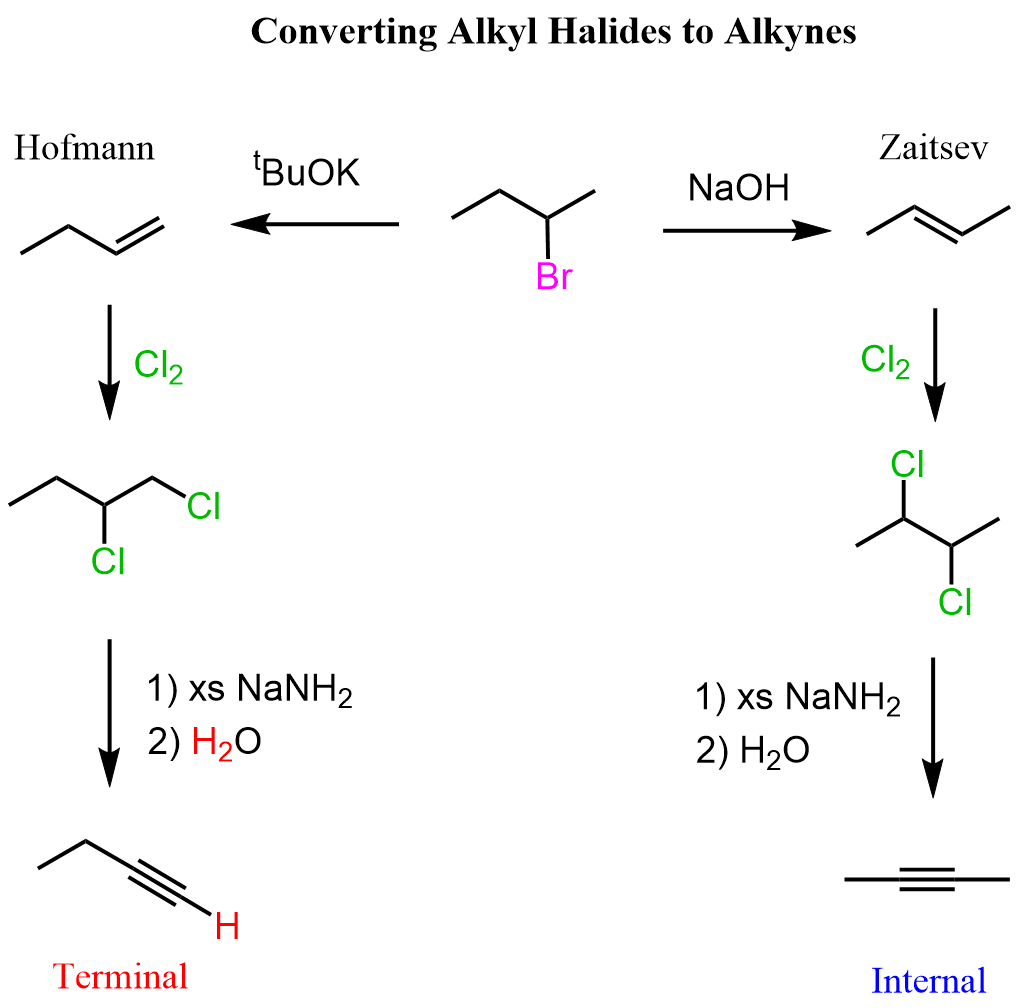
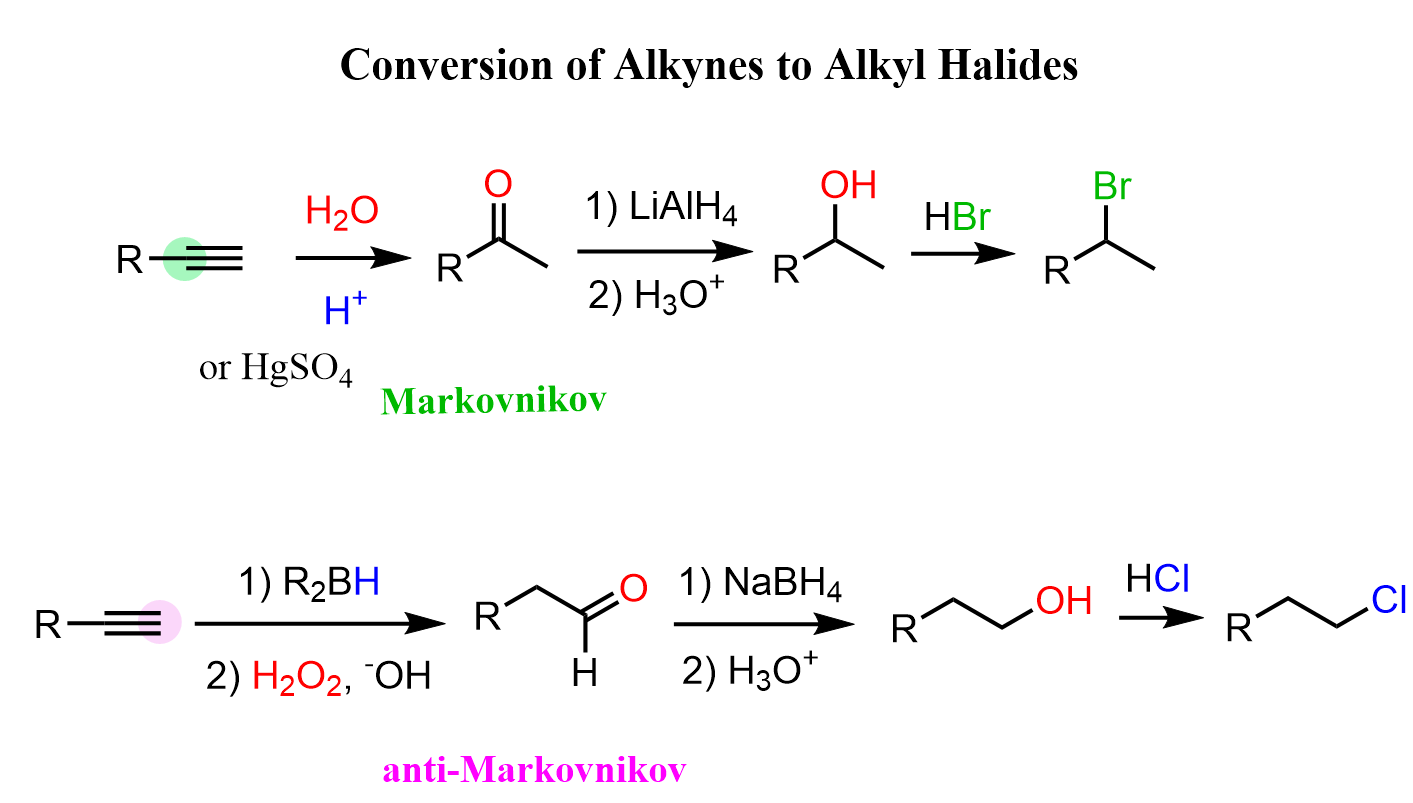
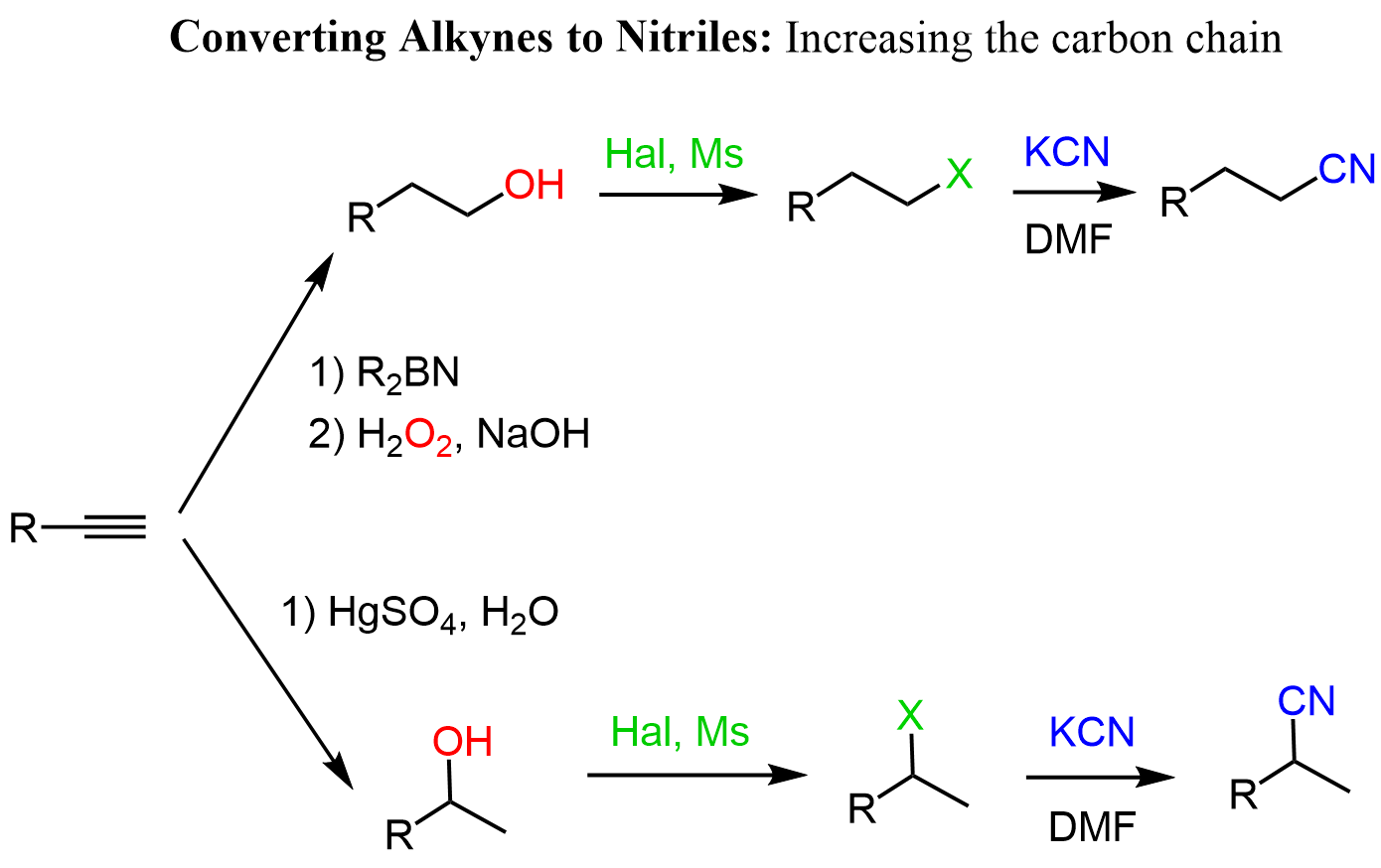
Acidity of Terminal Alkynes
Like any other hydrocarbon, alkynes are much less acidic than, for example, alcohols, thiols, carboxylic acids, and especially hydrohalic acids. However, what sets them apart from other hydrocarbons, such as alkanes and alkenes, is their relatively high acidity. … Read more