Decalin is the common name for fused bicyclic compounds that consist of two six-membered rings. As you may remember from the discussion about bicyclic compounds, fusing two six-membered rings does not give a 12-atom system because there are two common atoms. So, there are 10 atoms, thus the name, bicyclo[4.4.0]decane:
What is interesting about fused rings is the two possible geometries where the hydrogens on the bridgehead carbons can be cis or trans:
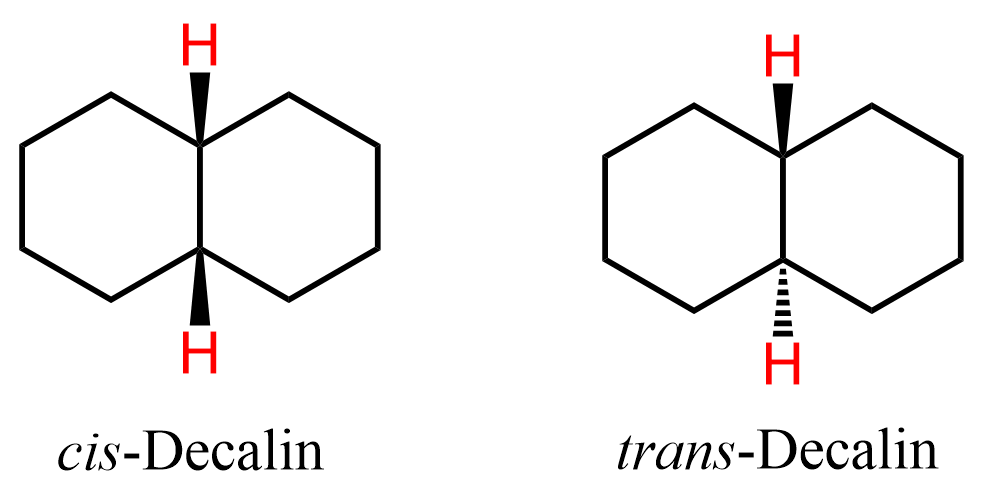
As we learned in the cis and trans isomerism of alkenes and cycloalkanes, when the two hydrogens are pointing in the same direction, we have the cis isomer, and when they are pointing in the opposite direction, we have the trans isomer.
The relative orientation of these hydrogen atoms is better seen when we draw the chair structure of the fused cyclohexanes:
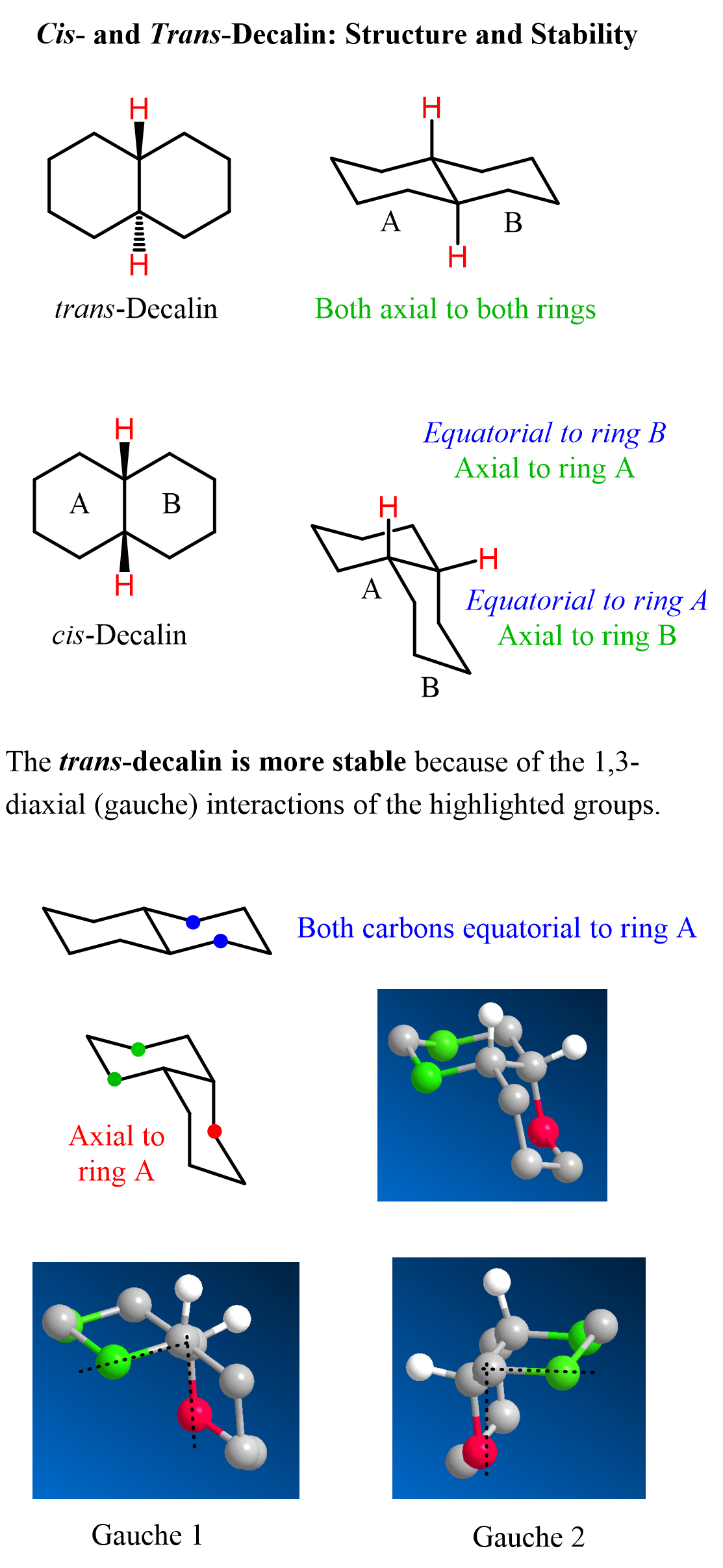
The trans isomer of decalin is more stable and by far more common than the cis isomer. So, why is it more stable? We can see in the image above that both hydrogens on the bridgehead carbons of the trans isomer are axial, and this may lead to a wrong conclusion about the stability of decalins. The hydrogens are colored only to show why the isomers are cis or trans. However, it is the carbon atoms that we need to pay attention to, as they are bulkier. Notice, in the lower part of the image, that one of the carbons of ring B is axial to ring A. This results in two gauche interactions with green carbons of ring A. Recall the 1,3-diaxial interactions in cyclohexanes:

If we delete all the carbons but the red of ring B, it will be easier to see the 1,3-diaxial (gauche) interactions with the green carbons of ring A.
What is the Relationship between cis and trans-Decalin?
It is important to know that these two cannot be interconverted via rotations of groups about carbon-carbon single bonds, and therefore, cis and trans decalins are not conformational isomers. They are stereoisomers, more specifically diastereomers, just like any other cis and trans isomers. You may also refer to them as geometrical isomers, especially if you have not covered stereochemistry and diastereomers in particular.
Ring Flip of Decalins
We know that cyclohexane interconverts from one form to the other via ring-flip. So, the question is whether decalins can undergo a ring flip. It turns out that the cis-decalin can, whereas the trans isomer cannot undergo a ring flip. Keeping in mind that when doing a ring flip, we keep the groups pointing in the same direction (up stays up, down stays down), let’s try to do it for both decalins:

As you can see, a ring flip of the trans decalin results in a structure with some unreasonable bond angles and lengths (I do not know for sure that this structure does not exist). The cis isomer, on the other hand, does not seem to look too crazy after the ring flip. The second ring is sort of like a half-chair, and if we twist the first ring a little bit, a nicer structure might be obtained. Master Organic Chemistry has a nice demonstration on this, too, so feel free to check that as well.
The Abundance of Decalins
Decalin systems appear in the structure of many naturally occurring compounds, such as estradiol, testosterone, and cholesterol. These are all steroids that incorporate three six-membered and one five-membered ring:

Note: The unique structure of decals leads to some interesting patterns in elimination reactions, which we cover in a separate post. If you’ve already studied the E2 mechanism and the requirement for antiperiplanar geometry, check the linked article for a detailed explanation and practice problems.
Check Also
- Cis and Trans Isomers
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems



Great