In the previous two posts, we have talked about drawing the ring-flip of chair conformations and the A value (1,3-diaxial interactions). And we learned that for a given cyclohexane, the axial conformer is less stable than the corresponding equatorial conformer. For example, the energy difference of the axial and equatorial isopropyl cyclohexane is 9.2 kJ/mol.

So, choosing the more stable chair conformation is straightforward when there is only one group on the cyclohexane. You just need to find the energy value for the axial group:

However, if there are more groups on the cyclohexane, we need to take into consideration the 1,3-diaxial interaction of all.
Example: what is the most stable chair conformation of the following substituted cyclohexane?

To answer this question, we need to draw the two chair conformations and compare the energies of all the axial groups on each conformer.
1. Number the ring and draw any chair conformation of the compound:

2. Draw the second chair conformation (ring-flip-check this post if not sure):

And now the stabilities: For each chair conformer, add the energy of all the groups on axial position.

In the first conformer, we have two chlorines in axial positions, so the total steric strain is:
2.2+2.2 = 4.4 kJ
For the second conformer, the chlorines now are equatorial, and we only have one methyl group in the axial position. Therefore, the energy is 7.3 kJ/mol.
Out of two conformations, the one with lower energy is more stable. So, despite having two axial groups, the first conformer is more as two chlorines do not bring as much steric interaction as the methyl group.


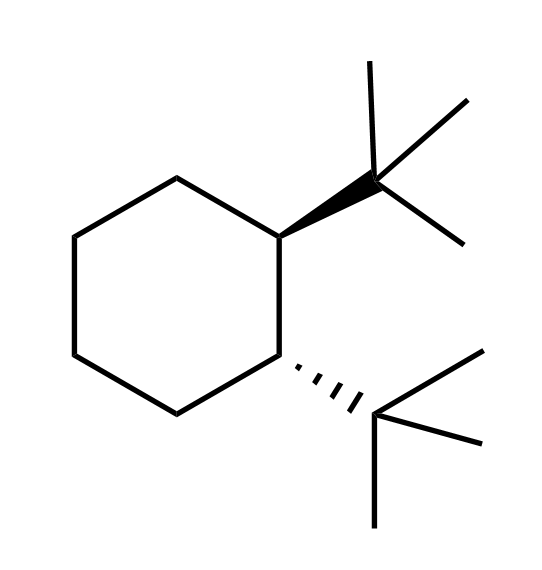
Isn’t the conformation more stable with bulkier constituents in the equatorial position?
Also in the video, I may be looking at it wrong but for question 1 shouldn’t the right side be more stable as it has lower energy? Plus it would be more stable because the ethyl group is in the equatorial position?
Hello,
Yes, having a large group in the equatorial position is preferred and it is also correct that lower energy means more stable.
However, we are not just looking at the bulkiest group and determine the stability of the conformation only based on its axial or equatorial position. You need to look at both conformations (ring-flip), find all the axial groups in each, and compare which one has a higher axial energy as a sum of 1,3-diaxial interactions of all the axial groups. So, in this case, two axial methyl groups have higher energy than one axial ethyl group even though one ethyl is bulkier than one methyl. Therefore, the conformation with the equatorial ethyl is less stable as it has two axial methyl groups.
Hi
Maybe that’s a stupid question but it’s not clear to me which substituents to draw axial and which ones to draw equatorial. From drawing the molecule, I don’t understand wich ones point away and which ones don’t…
Hi there,
No such thing as a stupid question. We are here to learn, and I encourage my students and everyone else to ask their questions.
For the position of substituents, you need to remember that the groups are not frozen in equatorial or axial positions. Cyclohexanes consist of single bonds only, with a constant rotation about them which means the ring carbons constantly change their conformation. This, in turn, means what is axial in one chair conformation, becomes equatorial in the other and vice versa. This is how the ring flip occurs. So, when you draw the chair, it does not matter if you put the first group axial or equatorial, but you must put the next group(s) according to what you decided for the first one. Whether the second group is axial or equatorial, depends on the first group, and how they are represented in the bond-line structure (wedge/dash) and wheter that corresponds to the axial or equatorial substituent position of the given carbon. Check the previous topic (Drawing Ring Flip of Cyclohexane) and feel free to ask any questions you may have.
Hello, I’m a bit confused. Did you not number your cyclohexane wrong in the 1st example? You numbered 1 from CH3 and proceeded clockwise but wouldn’t you number counterclockwise to have the substituents on lower numbers?
Hi Sam, it is a good point, and yes, if I were to name the molecule, I would need to make sure the substituents get the lowest possible locants. In the context of drawing ring flips and chair structures in general, it does not matter how we number the carbon atoms as long as we keep it consistent for both structures. So yes, you have a valid point, but the focus here was not on naming the molecule, otherwise, we’d have to start from the chlorine anyway to get the smallest set of the numbers – 1,2-dichloro-4-methylcyclohexane.