In the last few articles, we talked about the key electrophilic aromatic substitution reactions and the synthetic strategies based on the ortho, meta, para directing effects.
Below is a summary of electrophilic aromatic substitution practice problems from different topics. The answers can be found after the corresponding article.
Practice the Friedel–Crafts alkylation
1. Draw the mechanism for each Friedel–Crafts alkylation reaction:
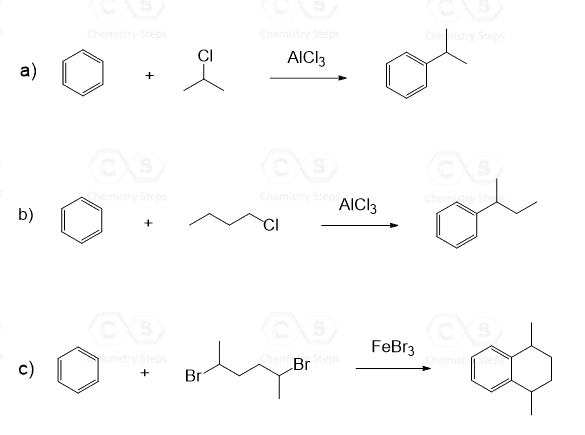
Check the Friedel-Crafts Alkylation post for more practice problems.
2. Determine whether each of the following reactions will proceed and predict the major organic product for each Friedel–Crafts alkylation reaction:
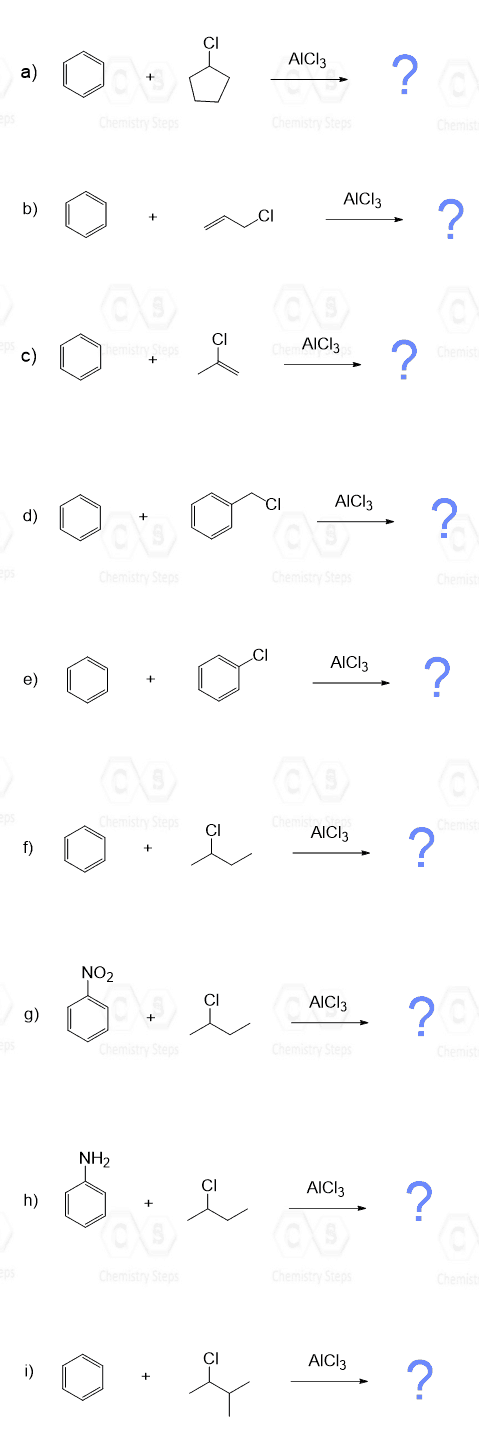
Practice the Friedel–Crafts acylation
Determine whether each of the following reactions will proceed and predict the major product, and draw the mechanism for the following Friedel-Crafts Acylation reactions:

Check the Friedel-Crafts Alkylation post for more practice problems.
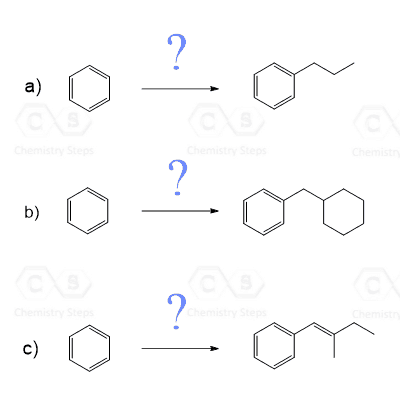
2. Show how each compound can be synthesized from benzene by using acylation reduction:
Ortho Para Meta Practice Problems
1. Classify each group as an activator or deactivator for electrophilic aromatic substitution reactions and mark it as an ortho– , para– , or a meta-director.
Print the table and fill it out as shown in the example for nitrobenzene.
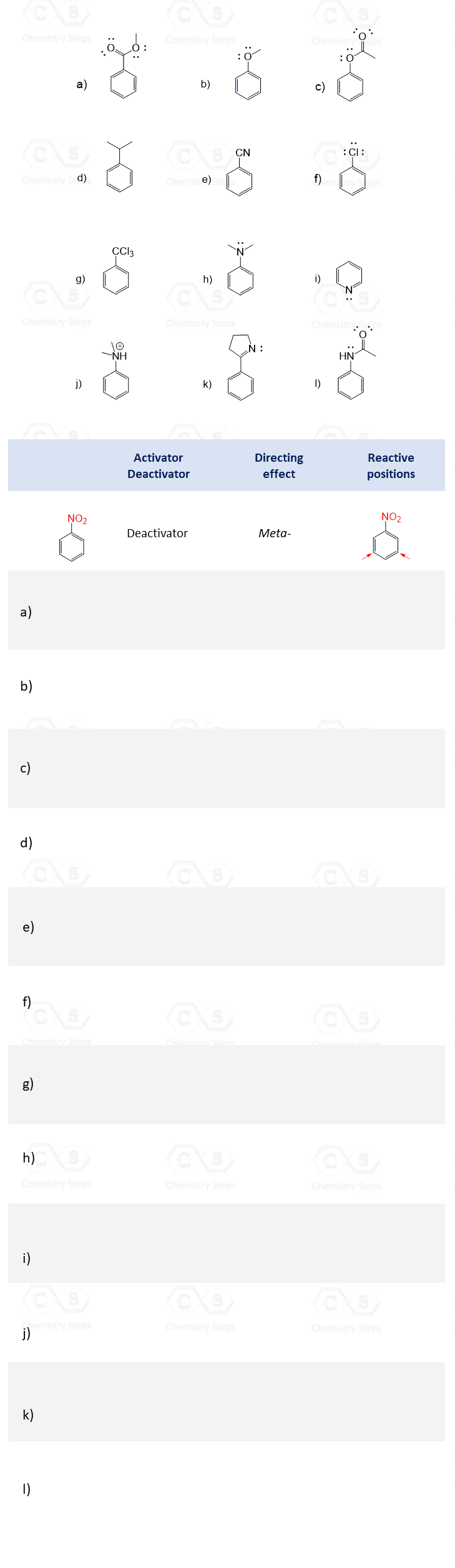
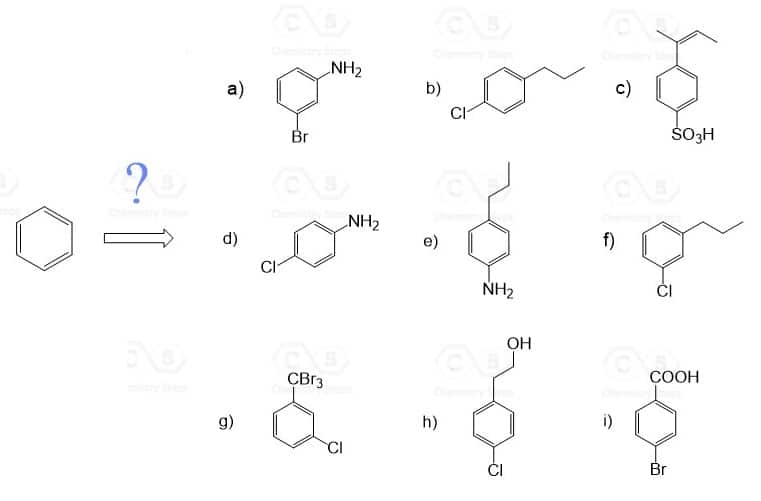
2. Identify the substituents as ortho- , para- or meta- directors and predict the major product for the following electrophilic aromatic substitution reactions:

3. Show how each compound can be synthesized from benzene and any other organic or inorganic reagents.
The order of reactions is very important! So, before every step, consider the ortho– , para– , or meta-directing effect of the current group on the aromatic ring.
Ortho, Para, and Meta in Disubstituted Benzenes
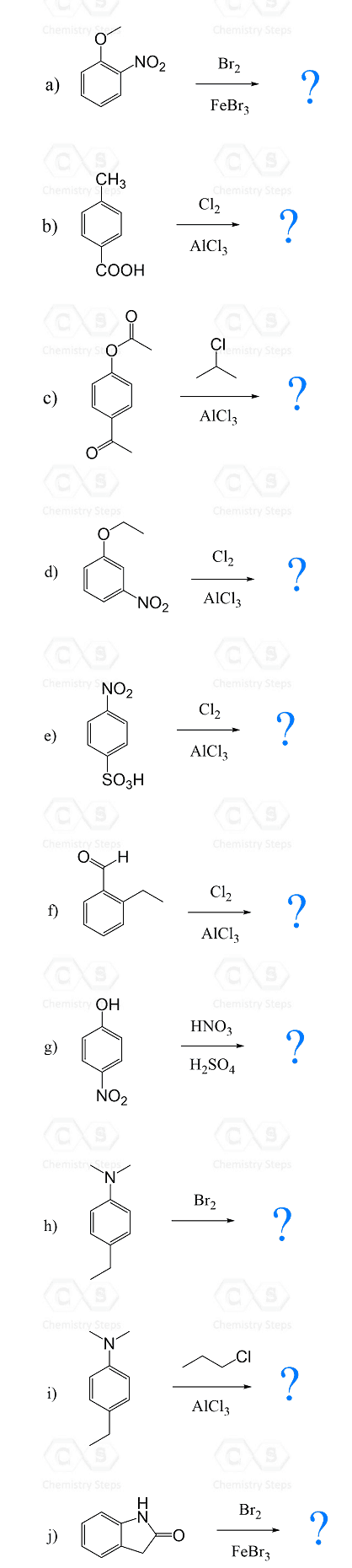
Predict the major product for the following electrophilic aromatic substitution reactions:
Hint: Identify the more active substituent and mark the reactive sides based on it first.
Show how each compound can be synthesized from benzene and any other organic or inorganic reagents.
The order of reactions is very important! So, before every step, consider the ortho– , para– , or meta directing effect of the current group on the aromatic ring.
Limitations of Electrophilic Aromatic Substitution Reactions
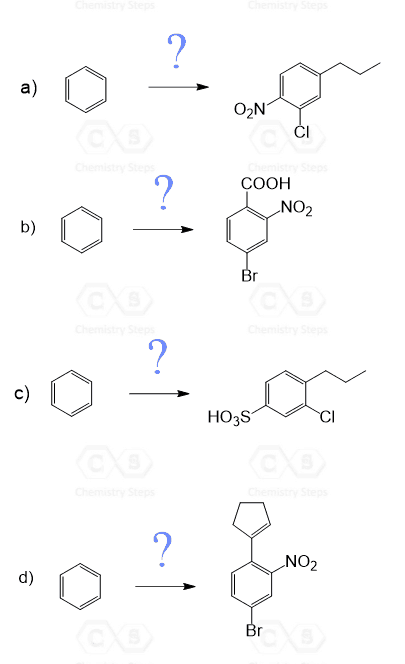
Determine which electrophilic aromatic substitution reactions will work as shown. Explain the reason for the ones that DO NOT work and show the other expected product (if any) for each reaction.

Arenediazonium Salts Practice Problems
Devise a synthesis of each of the following compounds using an arene diazonium salt. They all require more than one step, and you may select the desired regioisomer (for example, the para product from an ortho, para mixture) when needed.



Where are the answers? It just leads to another article.
Hi,
Which exercise is it?