In the previous few posts, we went over the basics of electrophilic aromatic substitution-halogenation, nitration, and sulfonation. Now, let’s dive into one of the most important EAS reactions discovered in 1877 by Charles Friedel and James Crafts, which allows for installing an alkyl group on the aromatic ring by making a new C-C bond: Friedel–Crafts Alkylation.
It uses an alkyl halide and a Lewis acid, typically AlCl₃, to generate a carbocation electrophile that the ring can attack.

The Mechanism of Friedel-Crafts Alkylation
It looks quite different from the aforementioned EAS reactions, so the natural question is, how does the alkylation of the benzene ring happen? Like many other electrophilic aromatic substitution (EAS) reactions—such as halogenation, nitration, and sulfonation, the Friedel–Crafts alkylation also requires the generation of a strong electrophile to be attacked by the nucleophilic aromatic ring. In this case, the Lewis acid AlCl₃ coordinates with the alkyl halide, pulling off the chloride ion and forming a reactive carbocation.
For example, let’s see how t-Butyl carbocation is formed when the Lewis acid polarizes the C-Cl bond of the alkyl halide:

Once the electrophile is formed, the π electrons of the aromatic ring then attack this carbocation, forming a non-aromatic sigma complex. Finally, a proton is lost, restoring aromaticity and completing the substitution to yield an alkyl-substituted aromatic compound:

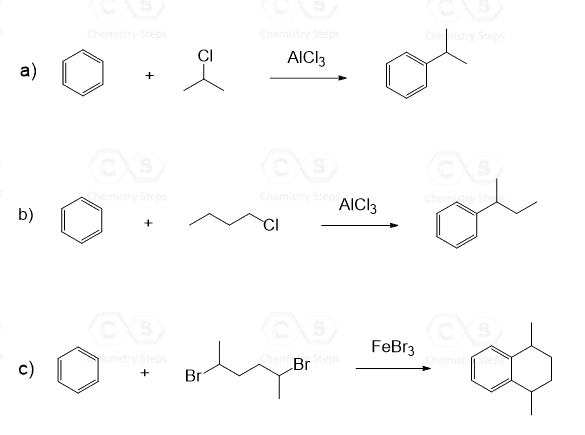
Notice that we used a tertiary alkyl halide to demonstrate the mechanism of Friedel-Crafts alkylation. There are a couple of reasons for this: 1) Recall that tertiary alkyl halides are “very stable,” 2) It is not all about the stability of the carbocation when it comes to the Friedel-Crafts alkylation. The carbocation must not be prone to rearrangement.
Rearrangements in Friedel-Crafts Alkylation
One of the biggest limitations in Friedel–Crafts alkylation is carbocation rearrangement. This may sound too basic to you at this point; however, recall that carbocations like to be as stable as possible, and when possible, they often undergo hydride or alkyl shifts to become more stable before reacting with the ring.
This means that even if you start with a primary alkyl halide, the product might end up with a secondary or tertiary alkyl group on the ring.
For example, how do we explain the following reaction, where we start with a primary alkyl halide, but the carbon connected to the aromatic ring in the final product is a secondary carbon?
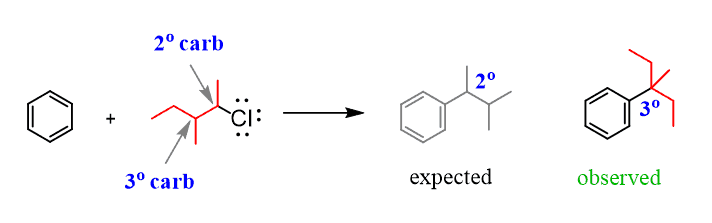
The following mechanism, showing a rearrangement of a secondary carbocation to a more stable tertiary carbocation, explains this observation:

Primary Alkyl Halides in Friedel-Crafts Alkylation
We know from nucleophilic substitution and elimination reactions that primary alkyl halides typically do not undergo SN1 or E1 mechanisms because the primary carbocation intermediate is far too unstable to form.
Surprisingly, rearrangement can occur even when a free primary carbocation isn’t formed. For example, the reaction between benzene and propyl chloride gives isopropylbenzene (cumene) as a final product:

So, how does this happen? When a primary alkyl halide reacts with a Lewis acid like AlCl₃, it forms a complex that makes the carbon more electrophilic, but doesn’t always produce a free primary carbocation. Instead, the complex may resemble a tightly associated ion pair or polarized structure.
Yet this “activated complex” is electrophilic enough that a 1,2-hydride or alkyl shift can happen before or as the aromatic ring attacks. This shift results in a more stable secondary or tertiary carbocation, which then undergoes electrophilic attack by the aromatic ring.

This is similar to what we have seen in the dehydration of primary alcohols:

Whenever possible, rearrangements are going to occur, and a mixture of alkylated rings will be obtained. The ratio can be optimized based on the conditions, such as the concentrations and temperature.
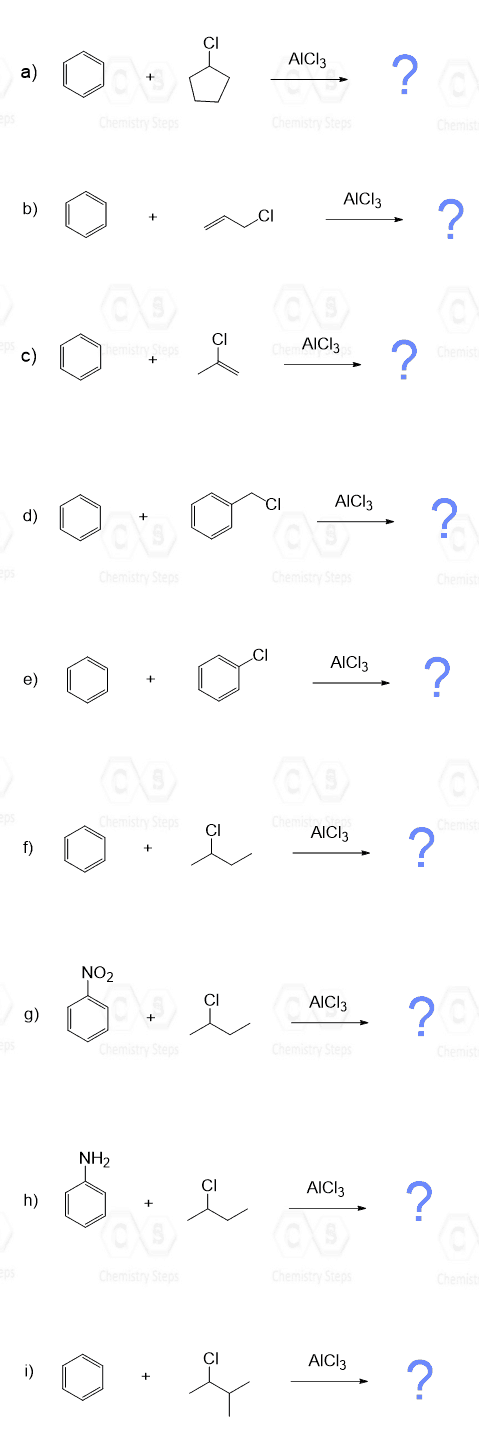
With this said, when is it possible to avoid rearrangements in Friedel–Crafts alkylation? Well, it will be the tertiary alkyl halides, as we have seen, plus the methyl or ethyl alkyl halides, since they cannot rearrange to more stable carbocations:
How to Avoid Rearrangements in Friedel-Crafts Alkylations?
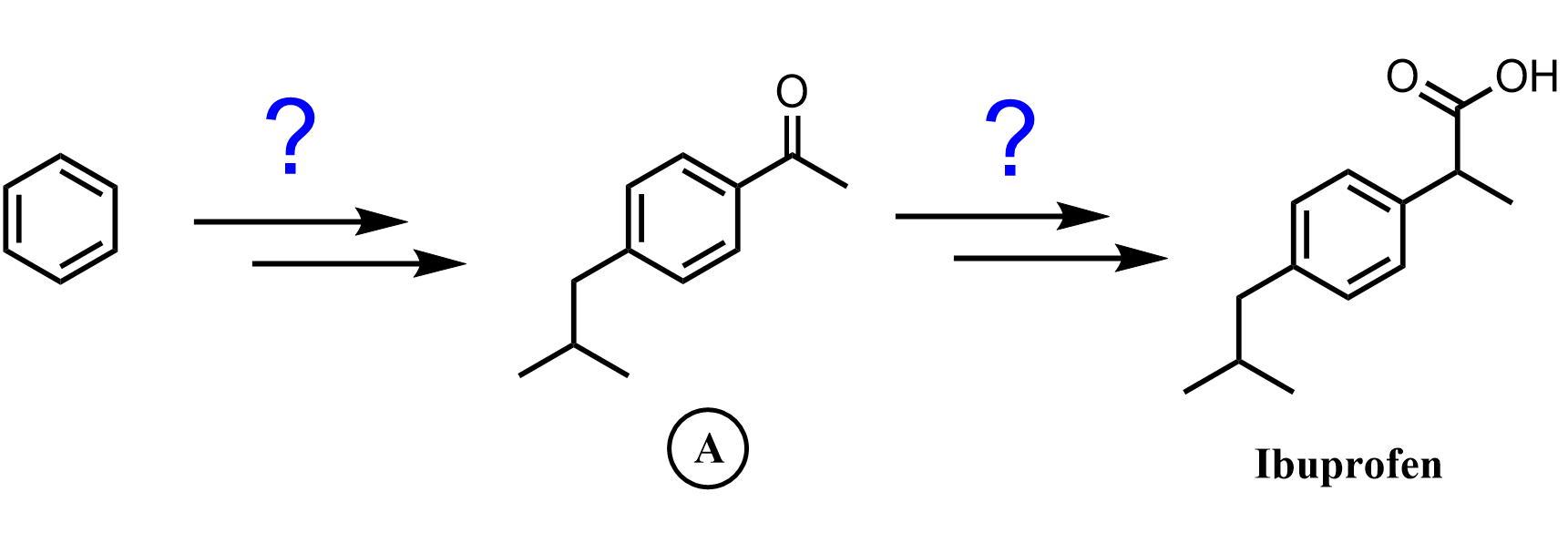
Aside from the clear restriction of using only methyl, ethyl, or other alkyl halides that can’t undergo rearrangement, there’s also the Friedel–Crafts acylation reaction, which is a valuable alternative. This reaction introduces an acyl group onto the aromatic ring to form a ketone. Importantly, the acylium ion, the key electrophile in this reaction, is resonance-stabilized and does not undergo rearrangement. That means we don’t have to worry about structural shifts like we do in alkylation. And as a bonus, the resulting aryl ketone can later be reduced to the corresponding alkyl compound, effectively giving a rearrangement-free path to alkylation.

We’ll save the details of Friedel–Crafts acylation for a separate post. For now, let’s focus on some of the key limitations of the Friedel–Crafts alkylation.
Limitations of Friedel–Crafts Alkylation
❌ Vinyl and Aryl Halides Don’t Work
For a Friedel–Crafts alkylation to proceed successfully, the halogen on the alkyl halide must be bonded to an sp³-hybridized carbon. This is because carbocations formed on sp²-hybridized carbons (such as those in vinyl or aryl halides) are highly unstable and don’t form easily under the reaction conditions. As a result, vinyl and aryl halides are unreactive in Friedel–Crafts alkylation and cannot be used in this transformation.

❌ Deactivated Aromatics React Poorly
If the aromatic ring has a strongly electron-withdrawing group (like –NO₂), it becomes too deactivated to undergo alkylation. In general, keep in mind that Friedel-Crafts reactions are the slowest among electrophilic aromatic substitutions, and they do not work when a strong deactivator such as NO2, CF3, and SO3H is present on the ring.
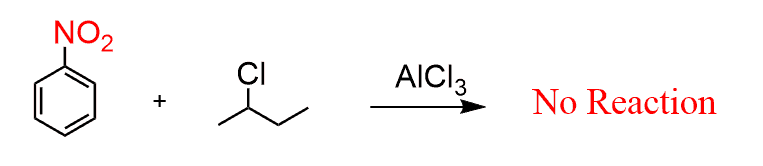
❌ Polyalkylation
Alkyl groups are electron-donating, which activates the ring for further reaction. This can lead to multiple substitutions, making it harder to isolate just one product.

Check out this article for more limitations and exceptions in electrophilic aromatic substitutions.
Other functional groups in Friedel–Crafts Alkylation
While alkyl halides are commonly used in Friedel–Crafts alkylation, they are not the only option. Any reagent capable of forming a stable carbocation can serve as the electrophile. For instance, alcohols can be protonated with H₂SO₄, leading to carbocation formation similar to E1 reactions. Likewise, alkenes can be protonated under acidic conditions to generate carbocations. Additionally, Lewis acids like BF₃ can activate alcohols or ethers, enabling them to participate in Friedel–Crafts alkylation.
For example, alcohols can be converted into carbocations in the presence of H2SO4, just like in E1 elimination reactions:

Alcohols can also be activated with Lewis acids such as BF3.
Alkenes are converted into carbocations in the presence of dilute acids (remember the acid-catalyzed hydration of alkenes), which serve as electrophiles in Friedel–Crafts alkylation reactions:

Intramolecular Friedel–Crafts Alkylation
If a molecule contains both an aromatic ring and a functional group that can be converted into a good electrophile, intramolecular alkylation can occur.

This strategy is useful for forming ring systems or fused aromatic compounds.
Summarizing Friel-Crafts Alkylation
Friedel-Crafts alkylation is a classic and versatile tool in organic synthesis, but you need to understand the nuances. Rearrangements, over-alkylation, and functional group compatibility all play major roles in how well the reaction works.
For more control and fewer rearrangements, you might want to consider Friedel–Crafts acylation followed by reduction, which we covered in our last post.