In today’s post, we will talk about the chlorination and bromination of benzene via electrophilic aromatic substitution. Remember, in the introductory post about aromatic compounds, we mentioned that, unlike alkenes, dienes, and other conjugated systems, benzene does not react with bromine.

No electrophilic addition to the double bonds of benzene occurs – at least not under the conditions when alkenes react readily.
Even a cyclic conjugated compound like cyclooctatetraene spontaneously reacts with bromine at lower temperatures:

The unreactive nature of benzene towards bromination, chlorination, and iodination is the special stability of aromatic compounds. Feel free to check the linked article for more details about this important feature of aromatic compounds.
To make the bromination or chlorination of benzene possible, the bromine is first converted into Br+, which is a strong electrophile and can be attacked by the p electrons of the aromatic ring.
This is achieved with a Lewis acid such as FeBr3, which helps to break the Br-Br bonds, producing the Br+ electrophile:

Once bromine is coordinated to FeBr3, an electrophilic complex is formed, and it is attacked by the electrons of the aromatic system according to a standard electrophilic aromatic substitution mechanism.

The same strategy is used for the chlorination of benzene, only AlCl3 is normally used instead of FeBr3, although either one will work:

Aromatic compounds with activating groups may be halogenated without the use of a Lewis acid catalyst. For example, aniline and phenol are so reactive to bromine and chlorine that the reaction cannot be stopped at monosubstitution, and 2,4,6-trihalogenation occurs:

Monobromination of phenol can be achieved at lower temperatures, which is more challenging with aniline.
You may not have covered this yet, but all the groups connected to the benzene ring are classified as activators and deactivators. Activators are those that make electrophilic substitution reactions (EAS) faster/easier compared to benzene, and deactivators are those that slow down or make certain EAS reactions impossible. Most activating groups are ortho, para, and deactivating groups are meta directors:

Notice that the halogenation and any other electrophilic aromatic substitution of phenol and aniline occur at the ortho and para positions because the OH and NH2 groups are activators. Check the linked article to read more about the reaction of phenol and aniline.
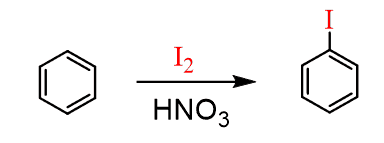
Iodination and Fluorination of Benzene
The iodination of benzene and derivatives requires using strong oxidizing agents that generate the I+ electrophile. The typical catalysts are silver(I) salts, HNO3, or a mixture of hydrogen peroxide and sulfuric acid.
N-iodosuccinimide (NIS) and N-bromosuccinimide (NBS) have also been shown to work for the iodination and bromination of benzene derivatives. The reagent ICl is a better iodinating agent than molecular iodine.
Fluorine is too reactive and thus impractical to use in many reactions, including aromatic substitutions. You can find more about the halogenation of arenes in March’s Advanced Organic Chemistry.
The Effect of Halogen on the Reactivity of Benzene
All the activating groups are ortho, para directors, and all the deactivators are meta directors. Halogens are the notable exception in this pattern as they are deactivators yet ortho, para directors. They are not strong deactivators such as the nitro and carbonyl groups, and many EAS reactions, including Friedel-Crafts alkylation and acylation, are possible to carry out on chloro- and bromobenzene.
For example, the sulfonation of chlorobenzene yields a mixture of ortho and para-substituted products:

Likewise, the halogenation of a halobenzene produces a mixture of ortho and meta dihalobenzenes:

In general, the ratio of the ortho and para substitution is difficult to predict. On one hand, the para position is favored because of the steric hindrance of the ortho position; on the other hand, there are two ortho positions and only one para, which statistically increases the yield of the ortho substitution. The larger the group present on the ring, the more favored the para substitution would be.
There is a separate article explaining why halogens are deactivators, yet they direct the electrophile to the ortho and para positions.
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds
