In simple words, the Hammond postulate predicts whether the transition state resembles the reactant or the product of the reaction. According to it, the transition state resembles the structure of the species (reactant, intermediate, or product), which is closer to in the energy diagram.
For a multistep reaction with intermediates and more than one transition state, Hammond’s postulate applies to each step, and we can predict the structure of each transition state. However, to keep it simple, we’ll start with a general scheme of energy diagrams followed by one-step reactions.
Recall that transition states (TS) cannot be isolated and characterized, and the only way to get an insight into their structure is by using computational theories (or Hammond’s postulate).
Reactions are either exothermic (more accurately, exergonic if we are talking about the Gibbs free energy) or endothermic, so let’s see how the Hammond postulate can help us determine the structure of the transition state. Here are two energy diagrams representing an exothermic and an endothermic reaction:
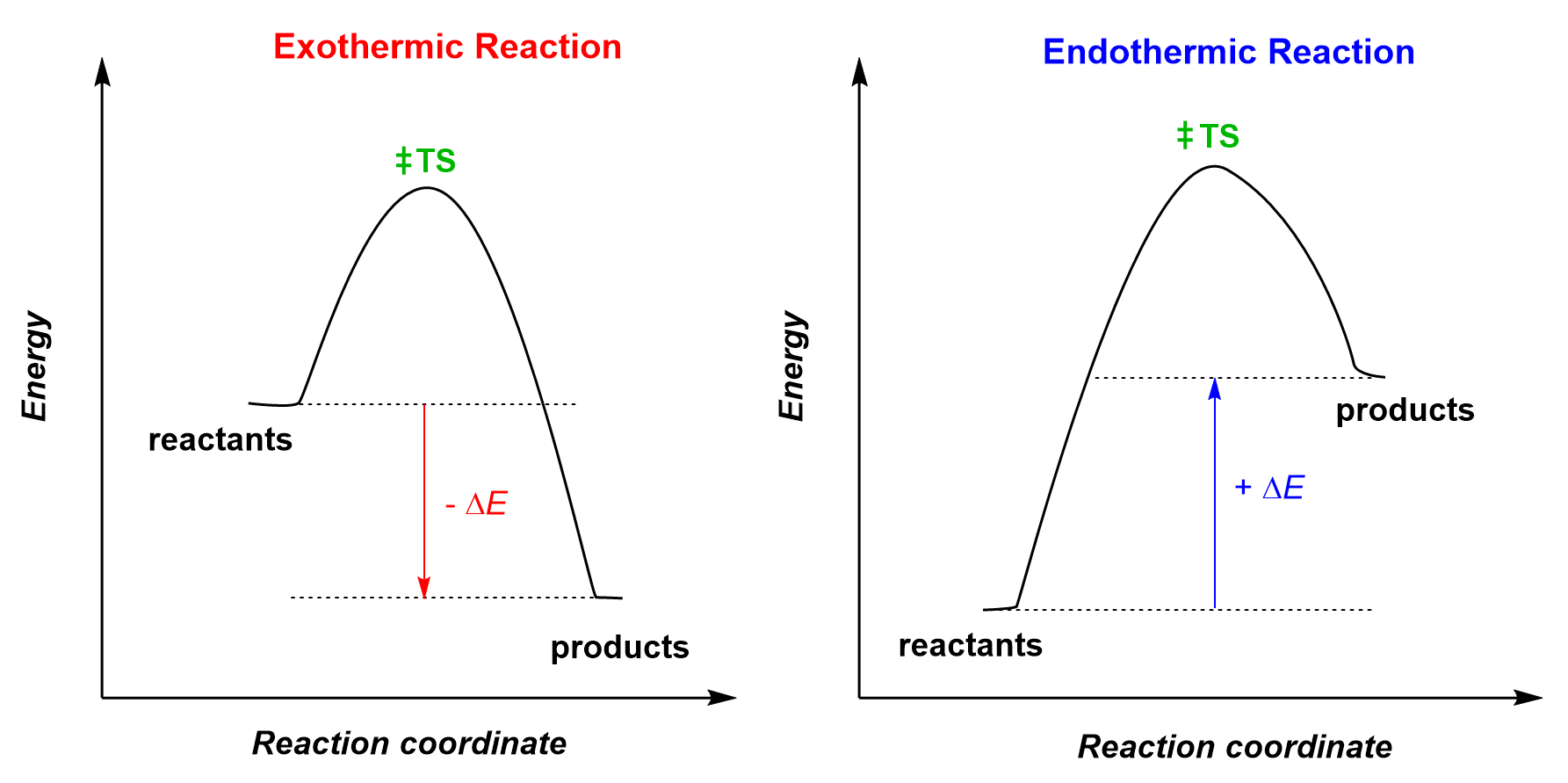
We notice that in the exothermic reaction, the transition state is closer in energy to the reactant, whereas, for the endothermic reaction, it is closer to the product. Therefore, according to Hammond’s postulate, we predict that:
- The structure of the transition state in an exothermic reaction resembles the reactant.
- The structure of the transition state in an endothermic reaction resembles the product.
For example, the following SN2 reaction between bromoethane and chloride ion is exothermic. You can see the calculations based on the bond dissociation energies to confirm that the reaction is exothermic in the linked article.
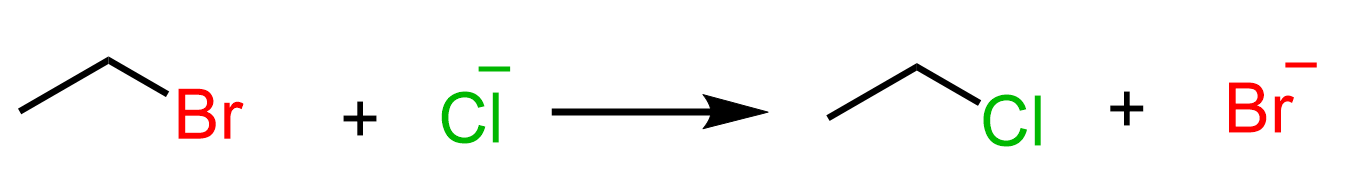
According to Hammond’s postulate, we predict that the transition state resembles more the reactant than the product:
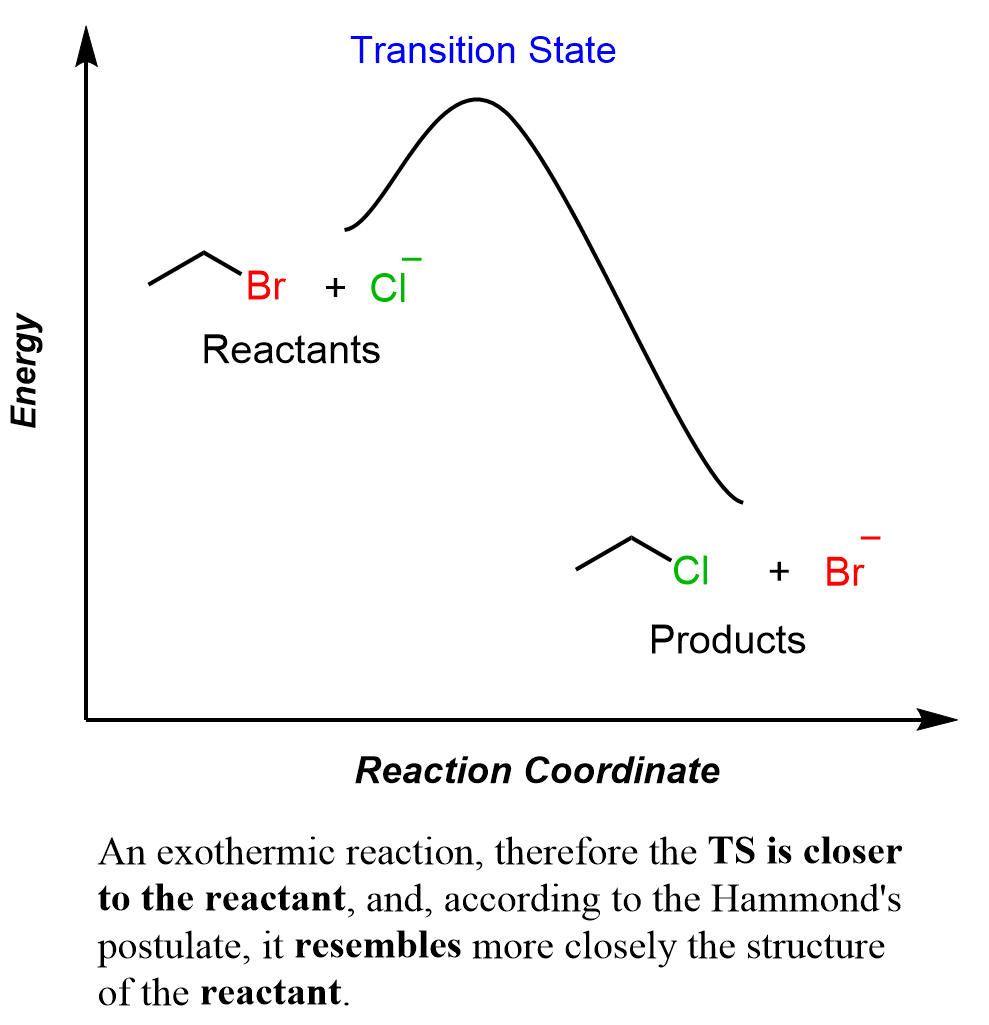
Now, what does this exactly mean? To answer this question, you need to remember that during the SN2 reaction, the bond between the leaving group (Br in this case) is breaking as the bond between the nucleophile (Cl–) is formed.
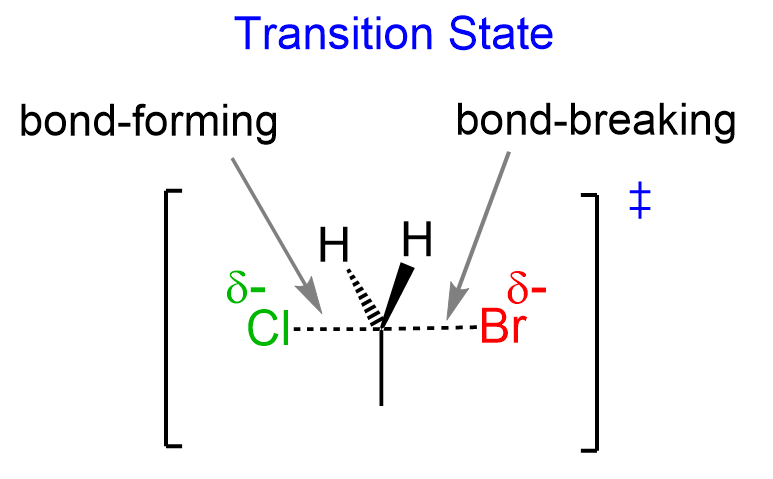
The breaking and forming bonds are shown with dotted lines, and the closer the bond to be formed, the shorter it is. On the contrary, the bond that is being broken is longer as the reaction proceeds.
So, for this reaction, we can say that in the transition state, the C-Cl bond is not as formed (longer dotted line) because the C-Br bond is still not as broken (shorter dotted line). We demonstrate this by making the C-Cl dotted line longer than the dotted line representing the breaking C-Br bond:

The full graph from the reactant to the product shows the entire journey of the reaction which is the breaking of the C-Br bond and the formation of the C-Cl bond. We can see from it, that the C-Br bond is mostly intact in the transition state as it is shorter than the newly forming C-Cl bond. Once again, the shorter the bond/line, the more it is complete, and the longer it is, the more broken it is. In the reactant, there is a C-Br bond and no C-Cl bond, and, as it is closer to the transition state than the product where there is a C-Cl bond and no longer a C-Br bond, the transition state is more like the reactant. This is the explanation, based on Hammond’s postulate, why the transition state of an exothermic reaction resembles more the reactant than the product.
Hammond Postulate for Endothermic Reactions
Let’s discuss the following addition reaction of HCl to 2-methylbut-2-ene, and see how Hammond’s postulate explains the formation of the more substituted alkyl halide (Markovnikov’s rule):
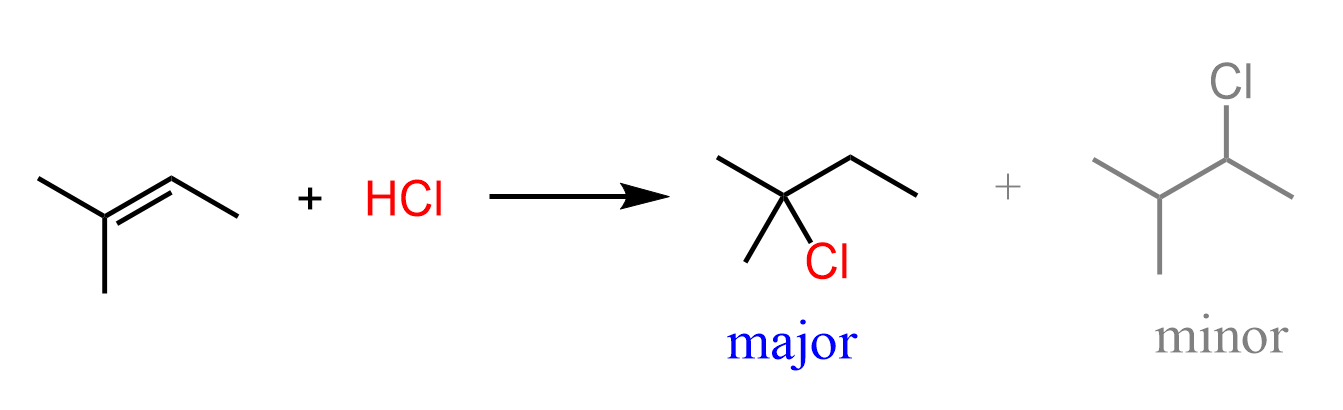
It is a two-step reaction that starts with the protonation of the double bond forming a carbocation followed by the nucleophilic attack of the chloride ion.
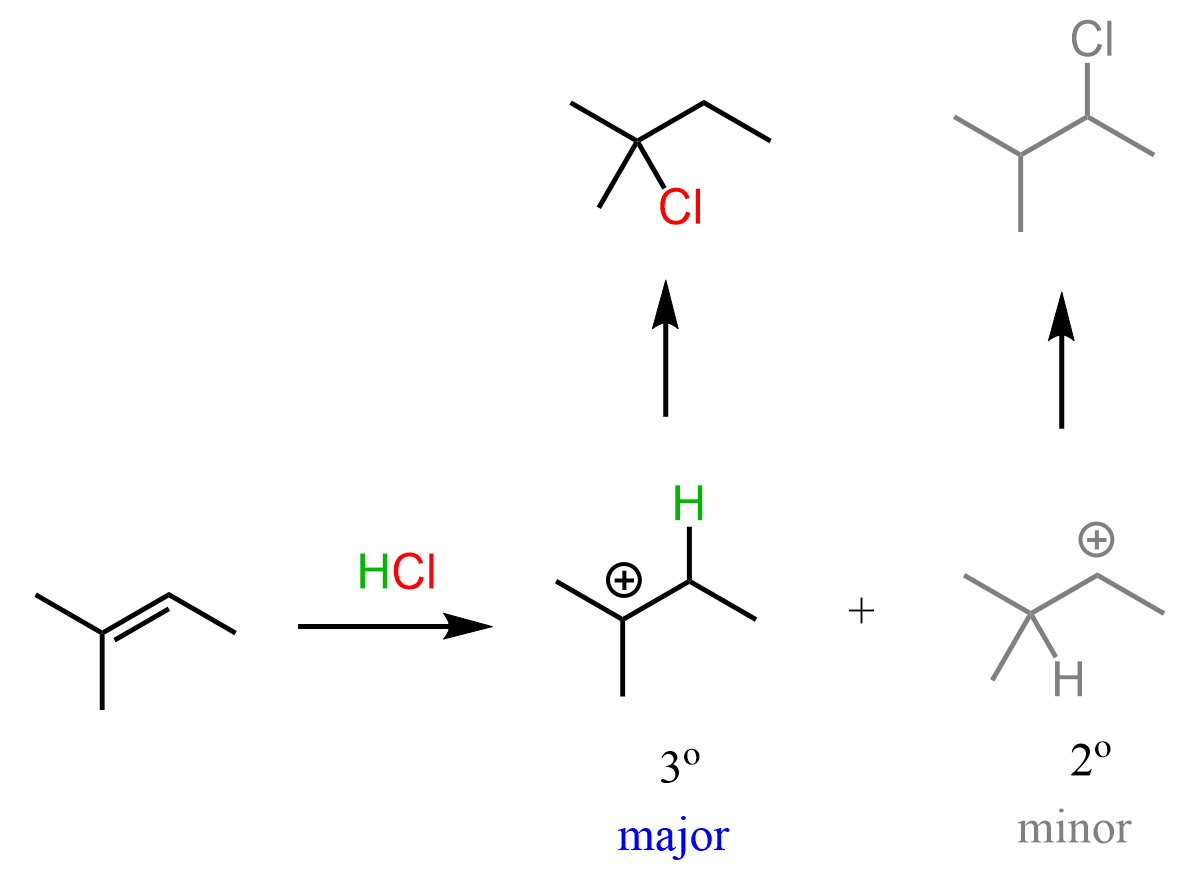
Overall, the reaction is exothermic, however, the first step, which is also the rate-determining step, is endothermic, and it determines the regiochemistry of the reaction.
Because of the greater stability, the addition of the protons forms a tertiary carbocation which leads to the corresponding alkyl chloride:
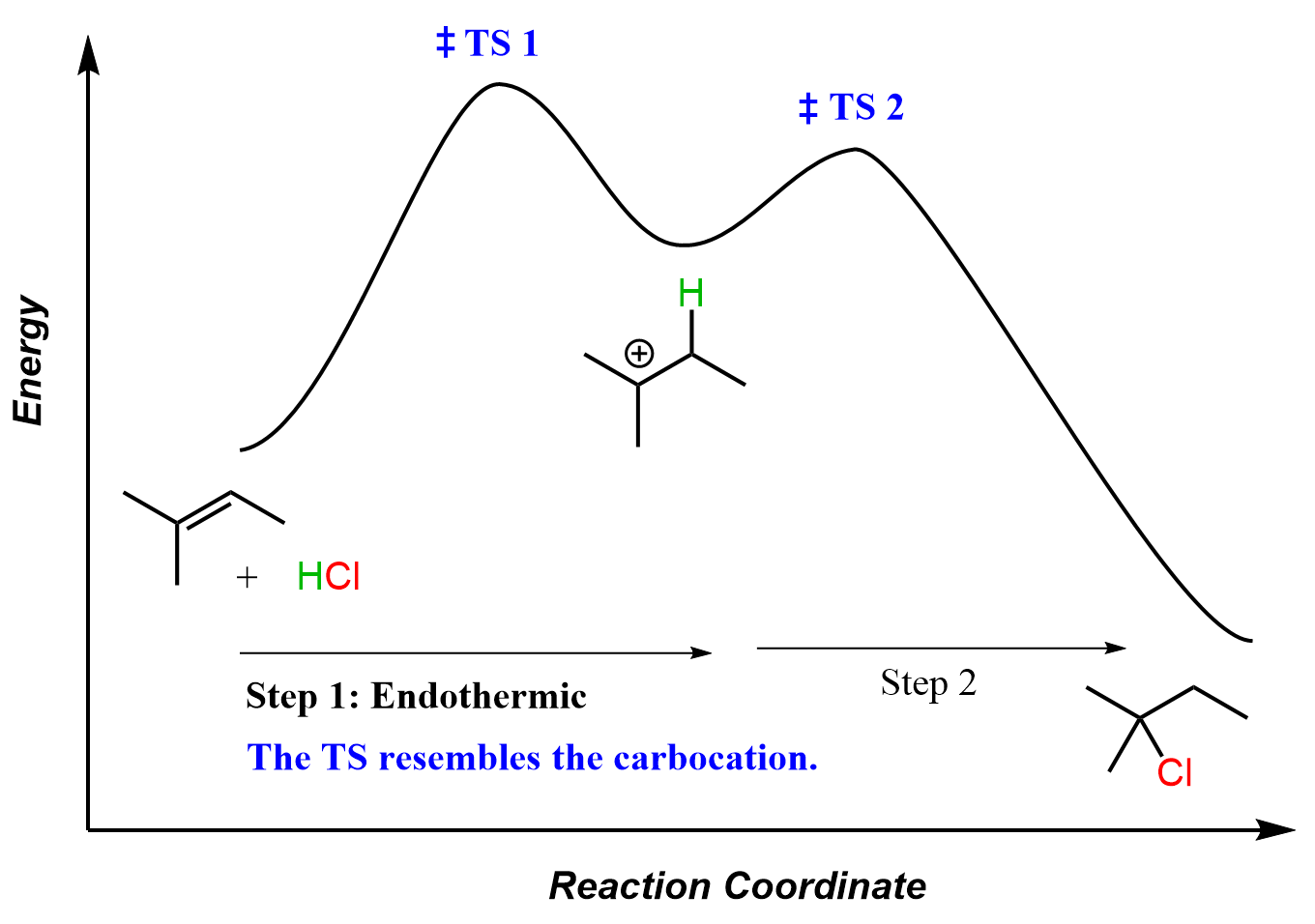
According to Hammond’s postulate, the first step is endothermic and therefore, the transition state resembles the structure of the carbocation more closely than the alkene. In other words, we can say that the C=C π bond is mostly broken in the transition state.
Knowing that the first TS is like a carbocation allows us to predict that it is lower for the formation of the tertiary alkyl halide than that of the secondary alkyl halide. And because the first step here is the rate-determining, we can say that the formation of the tertiary alkyl halide occurs faster, too.

Without relying on Hammond’s postulate, we couldn’t claim that the transition state resembles a carbocation, and therefore, we couldn’t argue that the TS of the reaction giving the tertiary alkyl halide must be lower (more stable) than that of the secondary carbocation.
In this sense, Hammond’s postulate served as a link between the stability of the product and the reaction rate.
Notice that this was for an endothermic reaction (step), and it is not a universal observation, especially for exothermic reactions, since the stability of the products does not affect the energy of the transition state so much. There are many reactions where the less stable product forms faster (kinetic product), and the more stable product forms slower (thermodynamic product). One such example, where we explain the reaction using energy diagrams, is the addition of HX acids to 1,2- and 1,4-dienes.
Check Also
- All You Need to Know About the SN2 Reaction MechanismThe SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The SN1 Nucleophilic Substitution Reaction
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions with Tons of Practice Problems
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- General Features of Elimination
- The E2 Mechanism
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- The stereospecificity of E2 Elimination Reactions
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism with Practice Problems
- Regioselectivity of E1 Reactions
- Stereoselectivity of E1 Reactions
- How to tell if it is E2 or E1 Mechanism
- Dehydration of Alcohols by E1 and E2 Elimination
- Mesylates and Tosylates as Good Leaving Groups
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- The Role of the Solvent in SN1, SN2, E1, and E2 Reactions
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
