What is a substitution reaction?
In a substitution reaction, one atom (or a functional group) replaces another one. The replacing group is called a “nucleophile,” and the group being kicked out is called a “leaving group.”:

These reactions occur because of the imbalance of the electron density between the carbon and halogen (leaving group) since it is a polar covalent bond. The more electronegative halogen pulls the electron density, thus making the carbon partially positively charged:
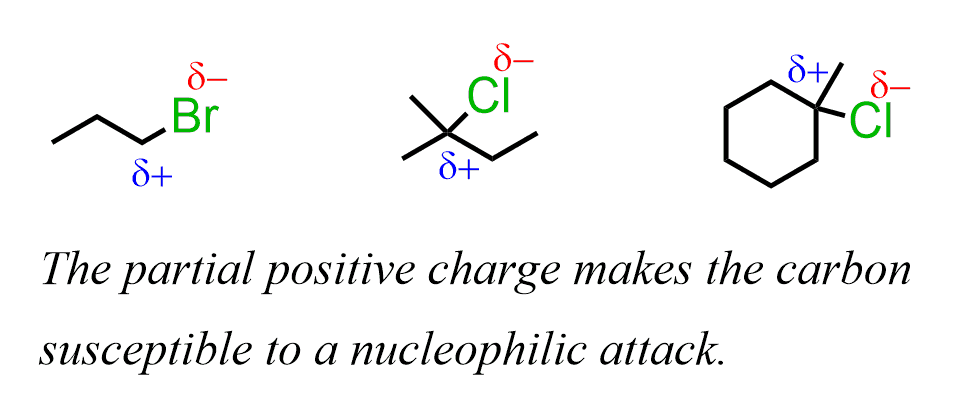
The carbon is, therefore, electrophilic – it is looking for some electron density to compensate for the partial charge. And, species with a high electron density are going to attack this carbon.
The molecule with the leaving group and the electrophilic carbon is called an electrophile. The most common electrophiles are the alkyl halides since the Cl–, Br–, and I– are good leaving groups.
Just like we call the carbon electrophilic, the species with a high electron density is called a nucleophile – “loving nucleolus”, i.e., loving a positive charge.
The Nucleophile and the Leaving Group
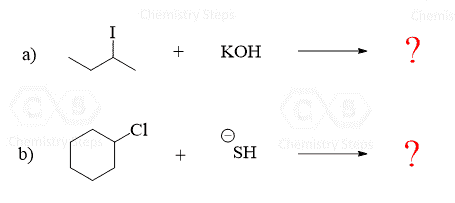
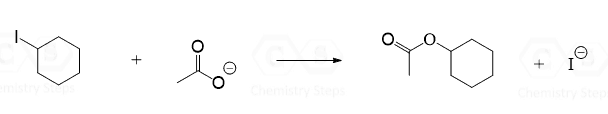
The nucleophile has to have at least one lone pair of electrons. However, very often it is going to be negatively charged since the negative charge makes it more reactive. Below are some common nucleophiles that you will encounter in your Organic Chemistry 1 class:
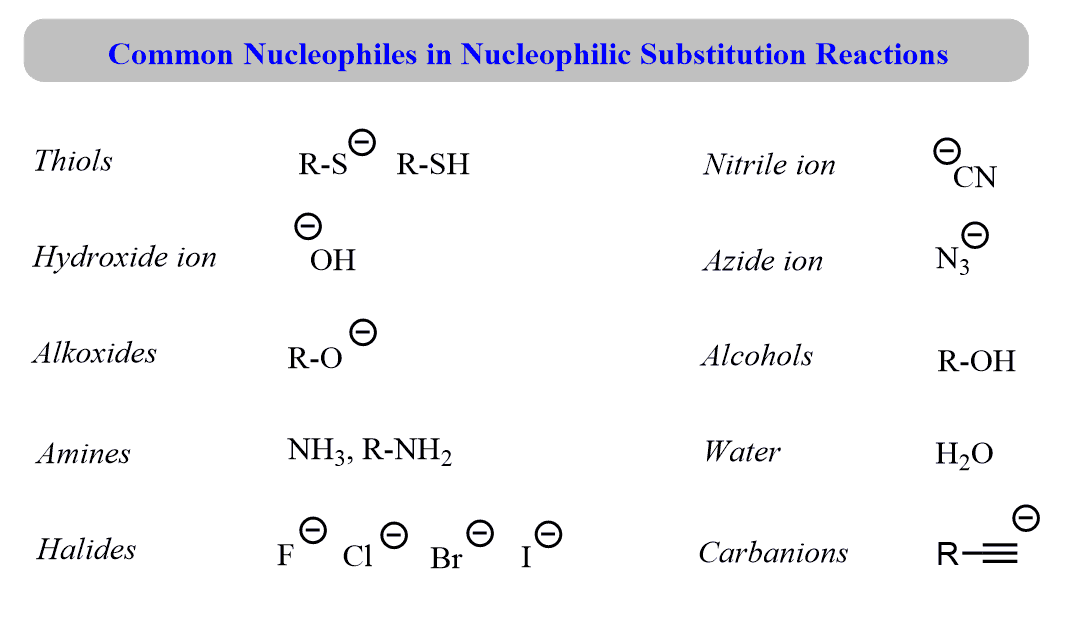
Having a nucleophile and a carbon atom connected to an electronegative atom is not enough for a substitution to happen. For example, the following reaction doesn’t work even though the carbon is connected to oxygen, which is a very electronegative atom:
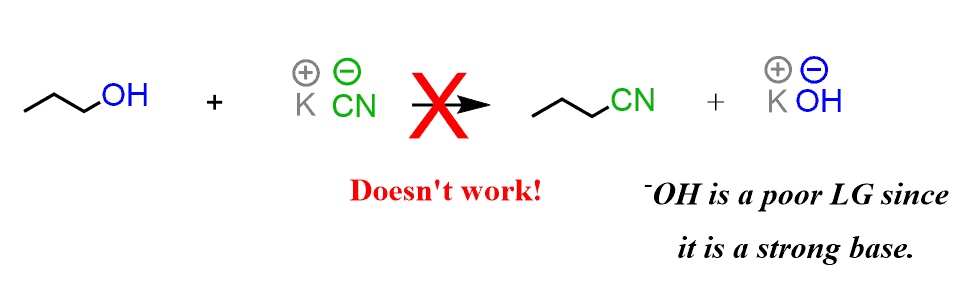
The trick here is that you always need to look at the leaving group (usually abbreviated as LG) as well. In order for it to be expelled by the nucleophile, it needs to be able to handle the negative charge well – it needs to be a weak base.
A good leaving group is usually the conjugate base of a strong acid.
Why is that?
Well, think about it this way: a strong acid is one with a large dissociation of the proton from the counterion (the conjugate base). So, the conjugate base is, in a way, a “leaving group”. The more stable it is, the stronger the acid.

You can read more about the acidity and pKa here.
The Mechanisms of Substitution Reactions
There are two main types of substitution reactions: One in which the nucleophilic attack and the loss of the leaving group happen at the same time, and the second, in which the loss of the leaving group happens before the nucleophile can attack.
When everything happens simultaneously, it is called a concerted mechanism. This is the SN2 mechanism.
When the processes happen in turn, this is called a stepwise mechanism – the SN1 mechanism.

In any case, you need to show the curved arrow starting from the nucleophile and point it exactly to the carbon (or without the LG if it is an SN1 mechanism).
Remember, curved arrows show the movement of electrons, and since the nucleophile is providing the electrons, the arrows should start from its lone pair or the negative charge.
After the nucleophilic attack, there is also a deprotonation step to form the alcohol. This proton-transfer step can occur both in SN2 and SN1 mechanisms.
![]()


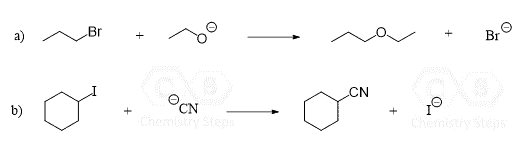




Is there a list anywhere that has the common reagents and solvents for substitution reactions?
Yes, you can find those in the following post comparing the SN1 and SN2 substitution mechanisms: When Is the Mechanism SN1 or SN2?
Similarly, this post will compare the E1 and E2 elimination mechanisms: How to Tell if the Mechanism is E1 or E2
If you have already covered both substitution and elimination reactions, read this article aimed at distinguishing SN1, SN2, E1, and E2 reactions.
The role of the solvent is also covered in a separate post: The Role of Solvent in SN1, SN2, E1, and E2 Reactions
Additionally, all of this is summarized in the 3-page substitution and elimination study guide.