Earlier on, when discussing the reactions of alcohols, we learned that the hydroxyl group is not a (good) leaving group, and one of the ways to address this problem was converting it into a mesylate or tosylate group. These are great leaving groups and allow for SN1 and SN2 substitutions when reacting with a variety of nucleophiles. The importance of the SN2 substitution is the control of the stereochemistry as it proceeds via inversion of configuration:

The Mitsunobu reaction allows for a one-pot replacement of the OH group by a nucleophile with an inversion of configuration. In other words, it is an SN2 reaction where we can replace the OH group directly without the need to convert it into a good leaving group:

The last sentence is perhaps an exaggeration because we do convert the OH into a good leaving group, but that happens in one reaction. That is the definition of a one-pot synthesis – it combines two or more steps that otherwise would require separate reactions for each step. So, how does this occur?
The Mechanism of Mitsunobu Reaction
Notice that there are two reagents in the Mitsunobu reaction we have shown above: triphenylphosphine (PPh3) and DEAD (diethyl azosicarboxylate):
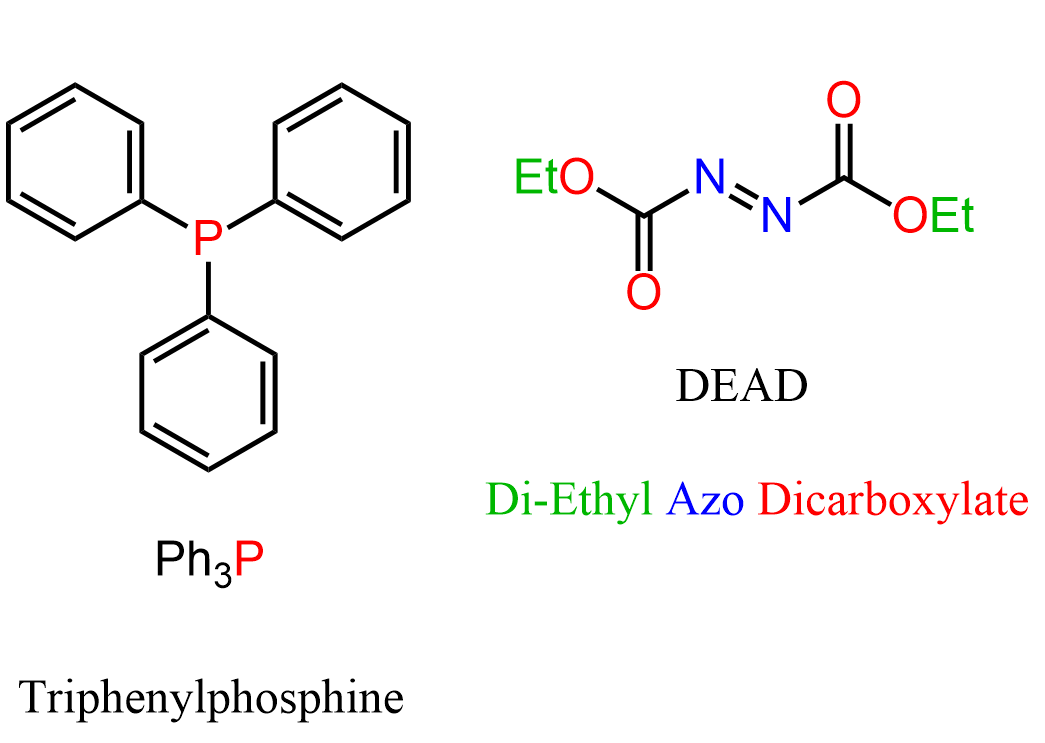
These two molecules are the magic combination for turning the OH into a good leaving group. Let’s see how it happens in the reaction of a secondary chiral alcohol with a carboxylic acid to produce an ester with an inverted configuration:

In the first step, PPh3, which is a great nucleophile, adds to one of the nitrogens of the weak N=N pi bond, forming a resonance-stabilized intermediate:
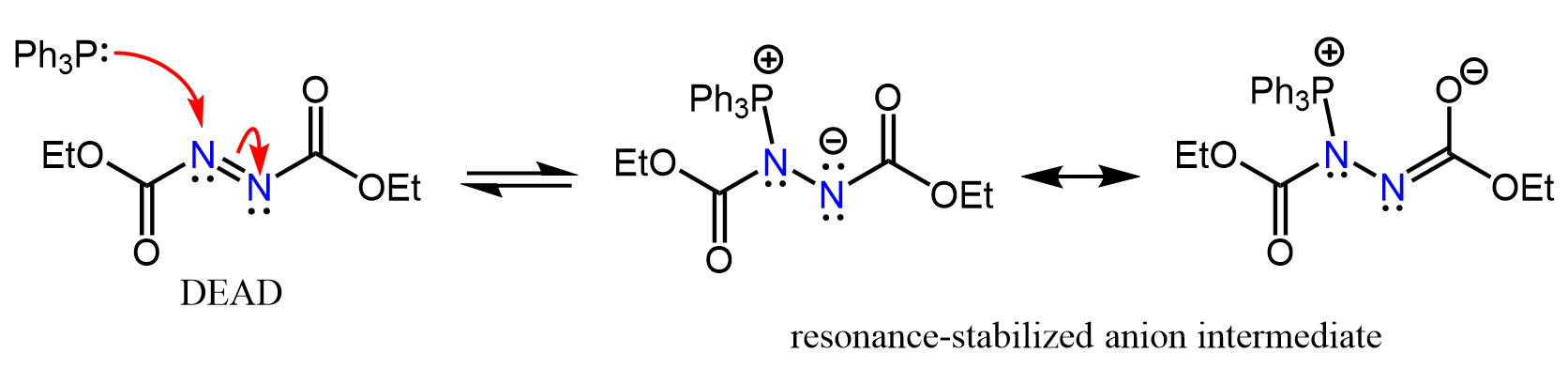
This anionic intermediate is basic enough to deprotonate the carboxylic acid and any proton within the range of pKa ≤ 16. Together with the carboxylate ion, another intermediate is formed with a positively charged phosphorus connected to a nitrogen:
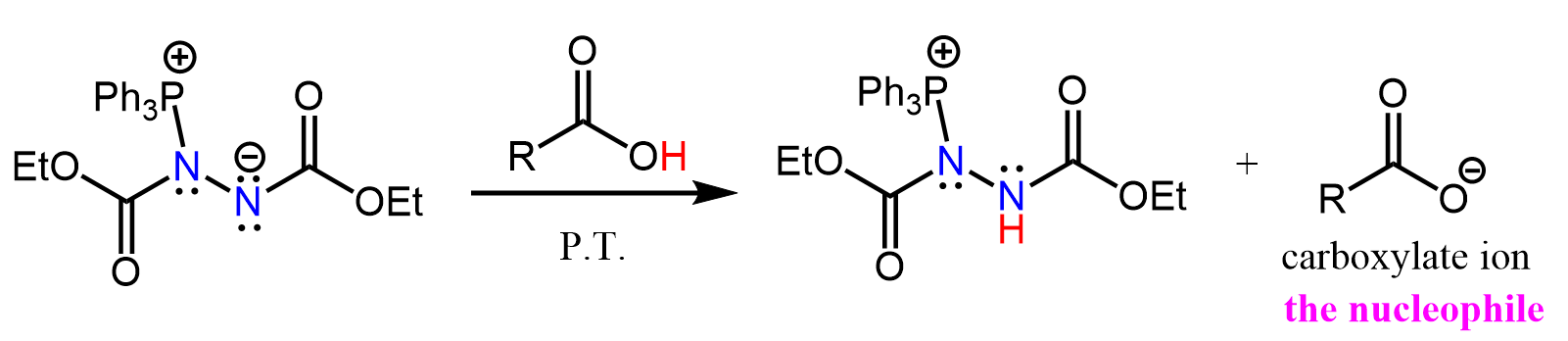
This phosphorus is then attacked by the alcohol, and after a proton transfer (P.T.), an activated alcohol (alkoxyphosphonium ion) susceptible to a nucleophilic attack is formed:

Recall that, despite being a weak base, the carboxylate ion is quite a good nucleophile and attacks the chiral center of alkoxyphosphonium ion by an SN2 mechanism to give the final product with inverted configuration:
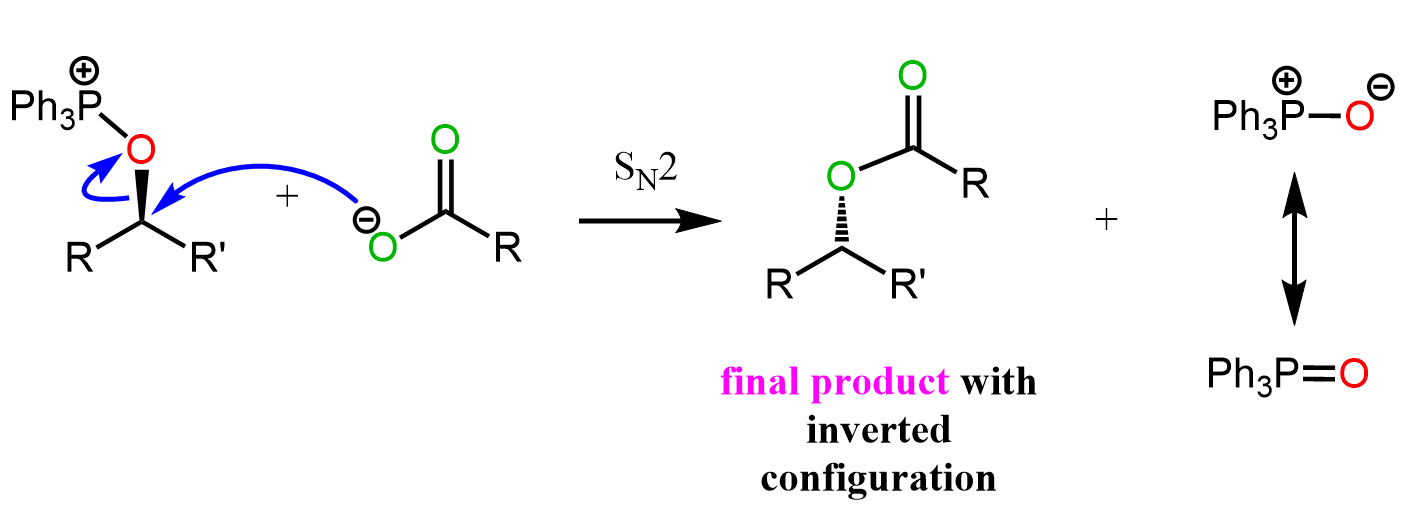
This was a lot of steps, but if we were to break down the Mitsunobu reaction, and compare it with the mesylation and tosylation strategies, we’d see that the only difference is how the OH is converted into a good leaving group. Instead of mesylates and tosylates, we now have an alkoxyphosphonium ion as a good leaving group:
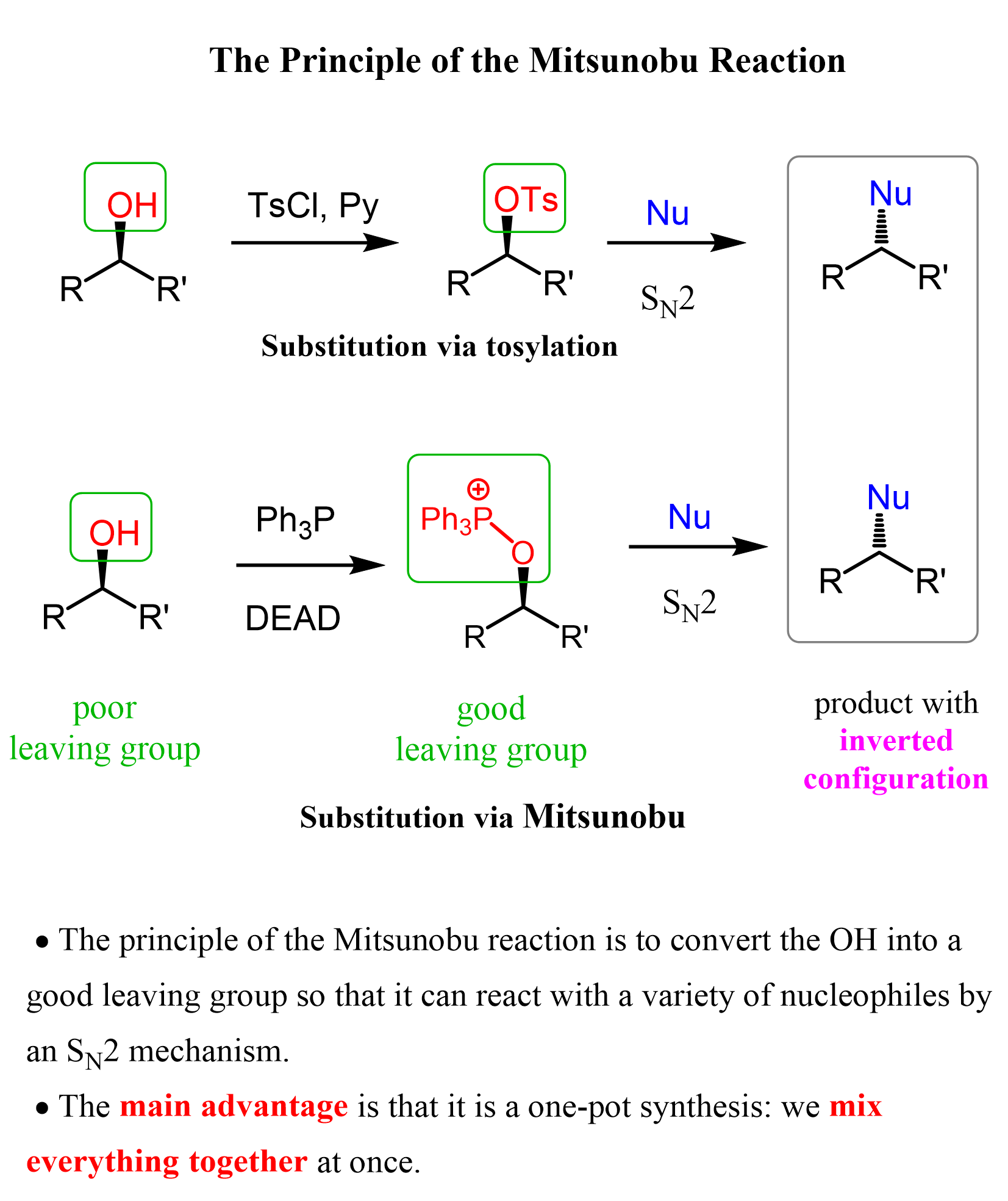
So, if one asks what the Mitsunobu reaction is used for, we summarize it as a strategy to replace the OH group with a nucleophile by an SN2 reaction. The main advantage of Mitsunobu reaction is that it is a one-pot synthesis: we mix everything together at once.
Going back to the reaction of the secondary alcohol with carboxylic acid forming an ester with inverted configuration, we can see that, unlike other esterification reactions, such as Fischer esterification, reacting alcohols with acid chlorides, the alcohol in the Mitsunobu reaction is the electrophile while in others, it is the nucleophile:

This is the reason for the inversion of the configuration when secondary chiral alcohols are used.
The Mitsunobu Reaction with Different Functional Groups
Let’s now see what nucleophiles can be used. As mentioned earlier, the nucleophile should ideally have a pKa ≤ 16 to make the deprotonation by the conjugate base of DEAD possible. Below is given a general scheme for how different nucleophiles are used in Mitsunobu reaction. For example, alcohols form ethers, carboxylic acids – esters, thiols – thioethers, etc.
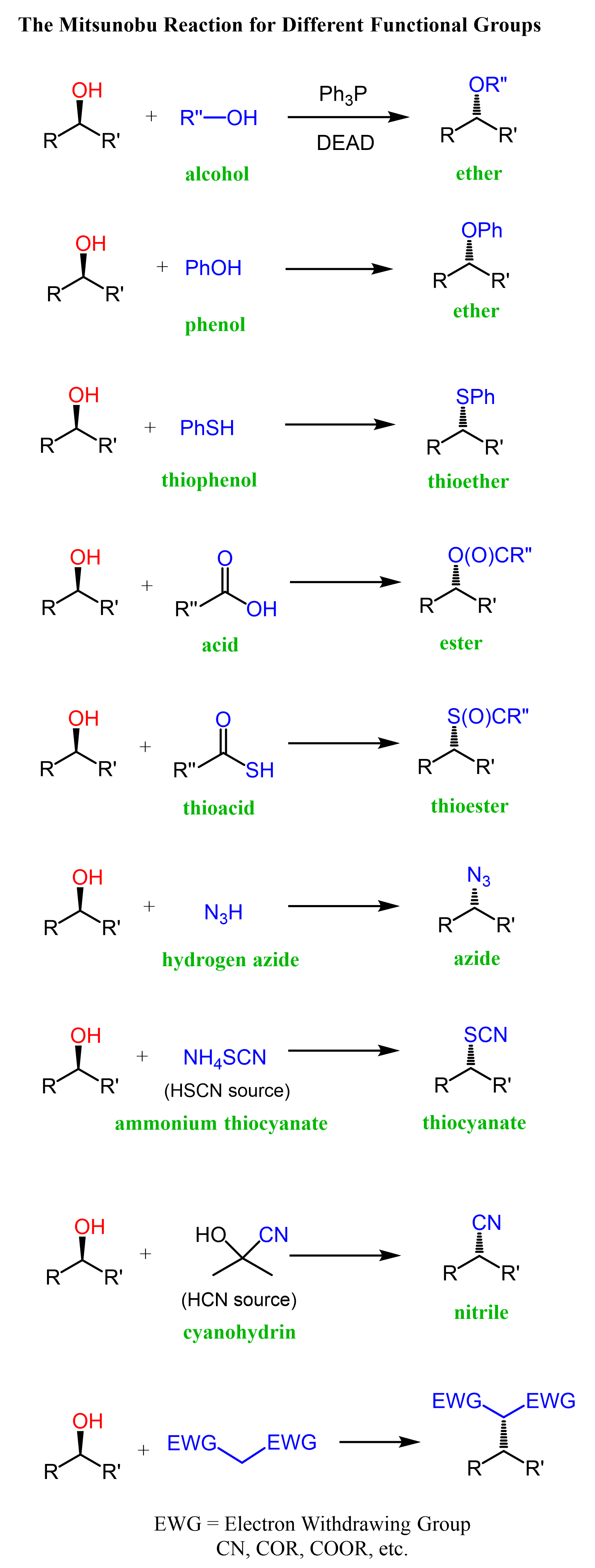
Notice that the reaction works for primary and secondary alcohols, and tertiary alcohols do not undergo the Mitsunobu reaction.
Epoxides in Mitsunobu Reaction
An interesting outcome is observed when epoxides are used in the Mitsunobu reaction. We know from their ring-opening reactions that good nucleophiles attack one of the carbons by an SN2 mechanism ,thus forming a product with an anti conformation. For example, azides open up the epoxide, placing the oxygen in an anti orientation:
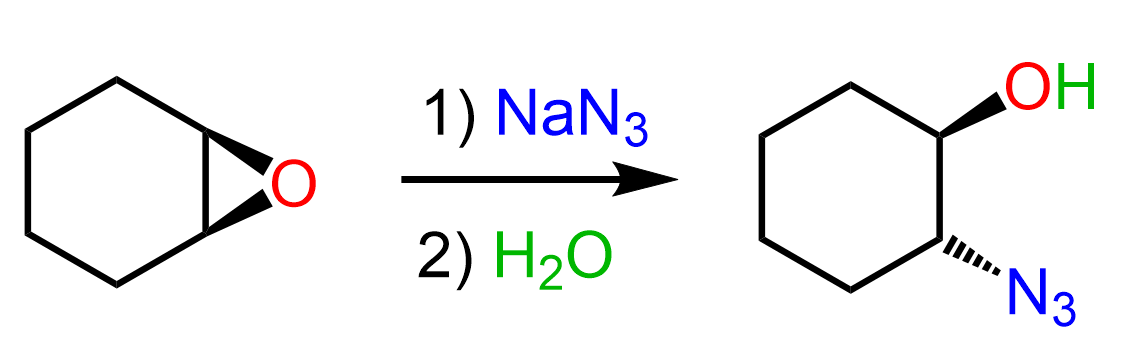
When an epoxide is used, two azide groups are added in a cis configuration:
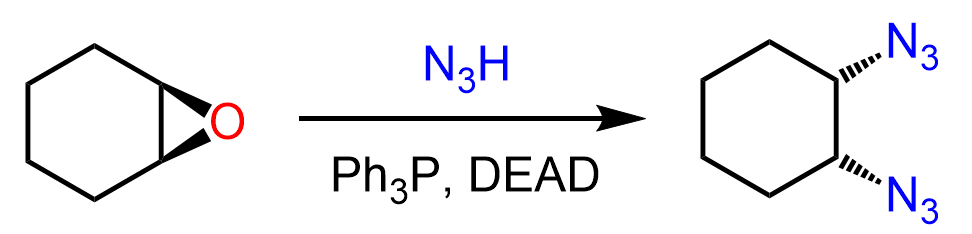
So, what happens is that after the first SN2 reaction, the anti product undergoes a Mitsunobu reaction, yielding a cis diazide:
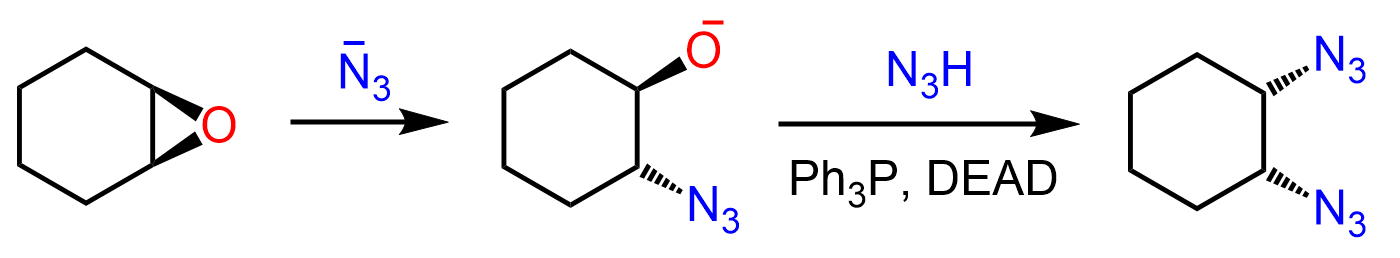
The azide ion can be formed from the deprotonation of the hydrogen azide by DEAD.
Intermolecular Mitsunobu Reactions
If the compound contains an OH and other functional groups, capable of acting as a nucleophile or helping the reaction to occur in any way, an intramolecular Mitsunobu can also occur. For example, a diol with a favorable number of carbons separating the two OH groups can undergo an intramolecular Mitsunobu reaction to form a cyclic ether:

Notice that we could have activated the secondary alcohol and gotten a different ether, but the primary alcohol is more nucleophilic and reacts with the PPh3 first. Also, if the secondary alcohol was activated, it would have been more sterically hindered to be attacked by the primary alcohol.
Mitsunobu Reaction with Retention of Configuration
An interesting example of a Mitsunobu reaction with retention of the configuration is the synthesis of Azidothymidine (AZT)/Zidovudine (ZDV)/Retrovir/Retrovis, a drug approved for the treatment of HIV. In the first part, the OH group is activated with DEAD and Ph3P, which facilitates the intramolecular attack of the nucleobase carbonyl, forming a cyclic ether structure. This is the first SN2, which inverts the configuration of the chiral center, and the second SN2 occurs when the azide nucleophile attacks and opens up the cycle. Thus, the configuration is inverted twice, which means it is retained:
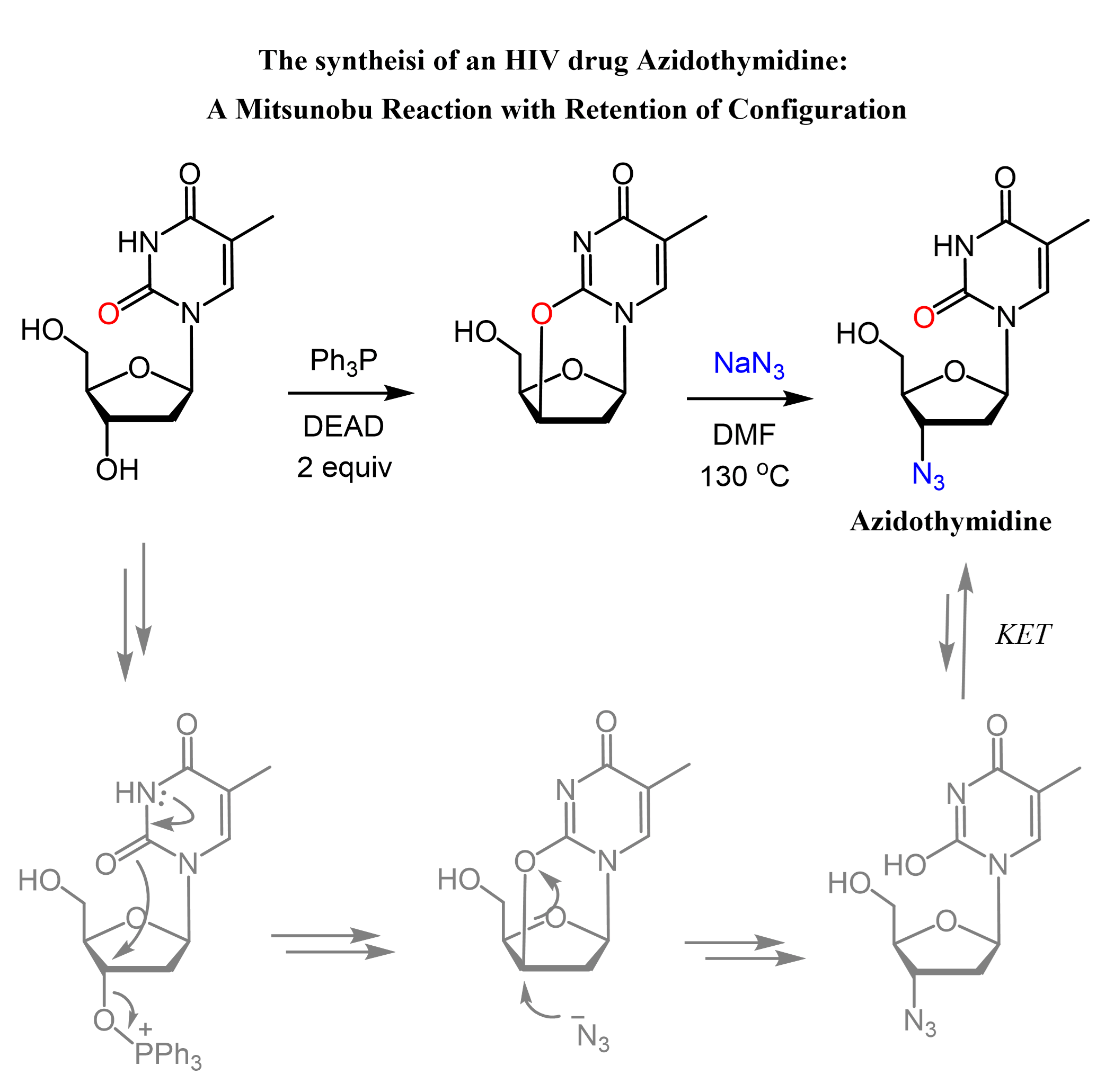
Notice that in the last step, the molecule is in an enol form, and it interconverts to the more stable keto form via keto-enol tautomerization (KET).
In the end, let’s put the Mitsunobu reaction mechanism in one scheme:

References:
Swamy et al. Chem. Rev. 2009, 109, 2551–2651
Luan – Strategic Applications of Named Reactions in Organic Synthesis
Clayden – Organic Chemistry
Lewis – Advanced Organic Chemistry
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- SN1 vs E1 Reactons
- SN2 vs E2 Reactins
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides

Very good content